4. Discussion
4.1. Analysis of microclimatic living conditions on exposed tree trunks
Only if the mechanisms that determine the heat and humidity supply at the trunk surface are understood in depth, can the possible adaptation of the arthropods be understood in depth, too. These climatic mechanisms at the trunk surface are most clearly reflected in the trunk/macroclimate differences.
4.1.1. Causes of temperature differences between trunk and macroclimate
The strong changes of the macroclimate's temperatures were reflected and sometimes even intensified at the trunk surface. KRENN (1933), HAARLOV & PETERSEN (1952) and NICOLAI (1985) also report similar correlations as well as strong temperature increases on sun-exposed zones. Only one additional influence on such heat exchanges at the trunk surface has been investigated until now (KRENN 1933): In summer, south-exposed trunk faces heat up to a lower extent because the inclination of radiation is too steep. In the present multiple regression analysis this mechanism was reflected by a negative correlation of upheating to the temperature of the macroclimate.
Basically, the extreme temperature differences between trunk and macroclimate were favoured by the low degree of evapotranspirative cooling especially on trunk faces of lower water vapour pressure. Thus, incoming radiation energy was mainly converted into an upheating of the surface (sensible heat flux, KERSHAW 1985 for cryptogam substrates). Even the larger evaporation in summer (GEURTEN 1950) does still not result in a lower upheating (KRENN 1933).
However, tree types of similar evapotranspiration (i. e. similar trunk / macroclimate differences of water vapour pressure) heated up differently and vice versa. Such effects of tree types were obviously not due to evapotranspiration but could be due to (after KERSHAW 1985):
(1) A low albedo (reflectance): Upheating is strongest, when the least amount of infrared radiation is reflected: on dark bark, mainly of beeches, less of lime trees and least of oaks (NICOLAI 1986, ashes are not considered). This sequence however is almost opposite to the one found for temperature differences between trunk and macroclimate. Albedos should also be especially low on trunks dominantly covered by - darkish green - algae. Nevertheless, exactly such trunks hardly intensified thermic fluctuations of the macroclimate.
(2) A thick atmospheric boundary layer (inferable from a strong reduction of wind speeds on the surface of a rough body, HÄCKEL 1994): this increases the resistance to balancing heat fluxes and thus stabilises steep temperature gradients. And indeed, the different tree types showed largely equal sequences in their wind reduction within the microrelief as in their trunk/macroclimate differences of temperature. Lower gradients than expected from wind reduction occurred only (a) on limes (explicable by the shading effect of bulbous, shrubby outgrowths on the trunk) and (b) slightly on oaks with a strong epibiosis by algae. In the latter, the exceptional climatology might cause the epibiosis - not vice versa.
(3) A low surface/volume-ratio of smooth trunks (HÄCKEL 1994): this reduces the contact to the environment to which heat can be lost by convection. But in fact the gradients are strongest on trees with a rough bark - as mentioned above.
4.1.2. Causes of water vapour pressure differences between trunk and macroclimate
Changes of humidities at the trunk were tightly coupled to the macroclimatic fluctuations and could even exceed them. If water vapour pressures were higher near the trunk than at a distance of 2 m the trunk lost water vapour through efflux (in contrast to temperatures, where increases mostly reflect a gain of thermic energy through radiation, WESTERMANN 1936 for soils).
There are two possible explanations for these losses: transpiration of water that was taken up by roots, or evaporation. In case of transpiration, most water vapour should be lost when the water content of the trunk is highest (measurements by GEURTEN 1950, inference by KOZLOWSKI 1958), i. e. when the tree looses least water by transpiration from the leaves: at night, during moist weather, and in winter (in contrast to summer, GEURTEN 1950) or during the emergence of leaves (April, in contrast to the time of leaf abscission, KOZLOWSKI 1958). However, none of these patterns was actually found.
Spatially, water vapour loss through transpiration should be highest where the distance between conducting vessels and the atmosphere is shortest: (1) in the valleys of the bark and (2) in tree types with a lot of crevices penetrating the bark: on oaks and limes, less on ashes and least on beeches (GEURTEN 1950, limes are not considered). But the differences in water vapour pressures between trunk and macroclimate actually measured did not increase in the bark valleys (recorded as wind-sheltered microrelief zones). And the hygric effects of different tree species were almost inverse to the sequence expected according to the mentioned transpiration rates (measured by GEURTEN 1950).
Consequently, the prevailing mechanism of water loss was passive evaporation - even during the season of highest transpiration abilities. This is the first analysis of the mechanisms by which water vapour is lost at the surface of trunks under wind-exposed conditions (LARCHER, pers. comm.).
Influence of the environment on water vapour fluxes. In soils, evaporative water loss is known to depend on (MONTEITH 1964, VANNIER 1970, THAMM pers comm.): (1) the wind speed of the ambient atmosphere and its relative humidity (under a certain temperature) or, more general, its water vapour pressure; (2) the temperature gradient between ambient atmosphere and the soil surface (3) the availability of water at the soil surface, (4) the solar radiation at that surface. All these factors could now be confirmed for the substrate bark and under natural conditions (Tab. 2). The third factor is reflected by the effect of water accumulation on certain trunk faces as well as the effect of rainy weather. The fourth factor (solar radiation) is reflected by the importance of an increased temperature compared to the macroclimate. Additionally, moderate wind speeds facilitated evaporative water vapour flux (by reduction of the boundary layer thickness). Strong wind, however, reduced evaporation by cooling down the surface and by turbulences mediating the temperature gradients (mechanisms from KERSHAW 1985). This explains, why the wind-sheltered trunk zones did in general not evaporate less (as do wind-sheltered areas elsewhere, KERSHAW & LARSON 1974).
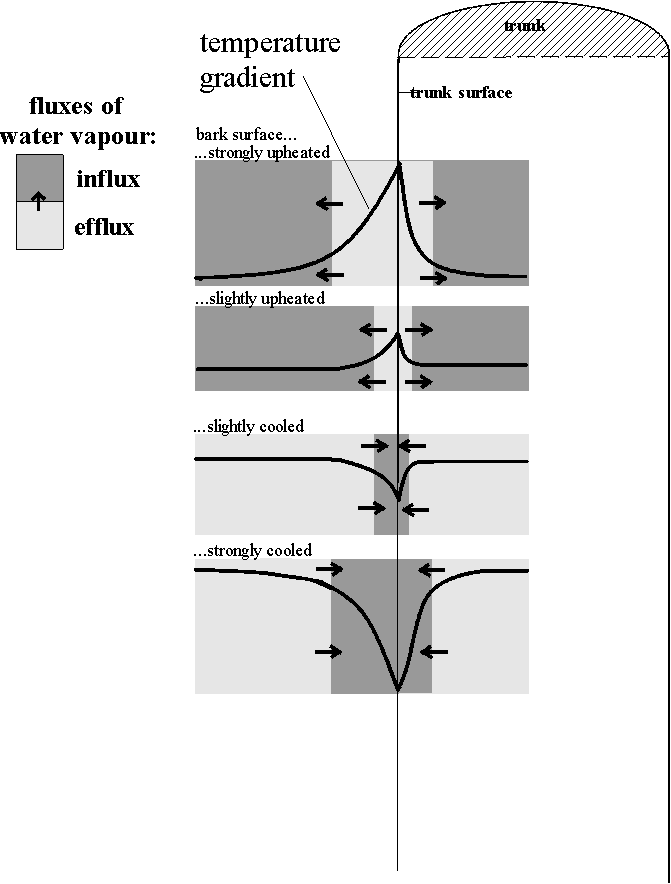
The humidity profile at the trunk surface. All possible mechanisms of evaporation/sorption induce and stabilise the following extremely small-scaled profile of water vapour fluxes at the trunk surface (Fig. 26): (i) an influx or efflux of water vapour towards/from only the outermost trunk surface during slight cooling or upheating of the trunk, (ii) an extension of the atmospheric layers involved during steep gradients. The possible mechanisms are: (1) condensation-induced source/ sink-dynamics: cooling of the trunk induces condensation and thus reduces the water vapour pressure, which attracts an influx of further water vapour from warmer layers (for soils: LAWREY 1991, KUNTZE et. al. 1994). The condensation is especially facilitated within the sculptured relief on bark or its cryptogam cover (mechanisms in MAIS 1970 for Collembola cuticulae). Below the trunk's surface, gradients of temperature will be basically inverse to those at the outer side of the trunk (HAARLOV & PETERSEN 1952 for sun-exposed trunk, GEIGER 1961, LAWREY 1991, STOUTJESDIJK & BARKMAN 1992 for any other kinds of bodies). This results in basically inversed water vapour fluxes (Fig. 26). (2) Absorption and desorption of water vapour by a cryptogam cover such as on trunk surfaces is especially quick (LERCH 1991) and absorption already occurs often in unsaturated air (KERSHAW 1985, HEATWOLE 1966). (3) Surfaces generally

Figure 26: Schemes of efflux and influx of water vapour at the trunk surface under different temperature gradients (for further explanation see text). The dimensions of a microarthropod and of its microhabitats accessible at different positions of the climatic gradients are given in the lower part of the figure.
exchange water vapour more easily because of the low capillary binding-forces there (VANNIER 1970, KUNTZE et. al. 1994).
Water vapour fluxes at outer surfaces of the trunk and of soils depend on similar processes, and they differ from the fluxes at leaf surfaces. Here, active transpiration is much more important, whereas an influx of water vapour has not yet been described (BURRAGE 1971, FERRO & SOUTHWICK 1984, WILLMER 1986). The rhythm of stomata opening thus creates severe but regular diurnal changes of humidities on leaves. This abiotic classification of habitats is in accordance with the faunistic compositions (GISIN 1943, AOKI 1971, LIENHARD 1976, WEIGMANN & KRATZ 1981, WUNDERLE 1992 a), which of course can additionally be influenced by factors like predation pressure and food supply. Soil and trunk behave differently, however, with regard to saturation deficits: the inner cavities of soil and litter are almost always saturated (VANNIER 1970), whereas the air in bark crevices can become unsaturated every day (LEWIS 1962). Thermally, trunks are more similar to leaves than to soils due to the small-scale thermal gradients (WILLMER 1986).
4.1.3. Conclusions on the climatic suitability of exposed tree trunks for arthropods:
4.1.3.1. Climatic disadvantages compared to alternative habitats
1) Climatic fluctuations at trunks are mostly more severe than at even the upper soil surface / the litter layer, despite of the slower heat exchange of the large trunk compared to the smaller, dry, dead leaves: Trunk temperatures always fluctuated > 10°C per month, up to 30 °C maximum, whereas at the ground, monthly fluctuations are below 10°C (VANNIER 1970, measurements at 0-5 cm soil depth; THAMM unpubl. results: in 16 out of 17 months, measured in the litter). Only on a seasonal scale did the litter temperatures fluctuate about as much as on trunks (VANNIER 1970, v. STRAALEN 1985, TAMM unpubl. results.). An upheating of litter to a degree similar to exposed tree trunks had only been recorded in an extreme case: on the uppermost surface of a large, dry tuft of grass litter on a sun-exposed hill in an open juniper shrubland during the short period of perpendicular sun radiation (STOUTJESDIJK & BARKMAN 1992).
2) Climatic gradients on the trunk were also often stronger than even at the litter / soil surface, despite the trunk's exposure to wind turbulences. The temperature differences within the microrelief reached up to 8.2°C, and between opposite trunk faces up to 22 °C. In contrast, the differences measured between litter and soil in 0-5 cm depth are less than 2°C (VANNIER 1970). Litter and the air at a height of 5 cm only differ less than 3°C (except of 11 days during a whole year, THAMM unpubl. results). Thus, in the litter zone, the available volume of suitable habitat is much larger for an animal once it has found a patch of favourable climatic conditions or once the animal has been born there.
Fluctuations and gradients of trunk climate are also extreme compared to those on bark within the tree crowns (BROADHEAD & THORNTON 1955), which is another alternative habitat for colonisers of trunk bark. Here, surface temperatures of dead branches in exposed and sheltered larch crowns hardly differ more than 2°C during July. The difference between the branch surface and air in 30 cm distance is equally low. Relative air humidities in exposed and sheltered crowns almost always differ less than 5 % (BROADHEAD & THORNTON 1955).
3) Optimal climatic shelters, being completely saturated with water vapour, were rare. They occurred primarily when a trunk face had both, lower temperatures and higher water vapour pressures than the opposite trunk face, and when the macroclimatic water vapour pressure was high while the temperature was low. Very often, however, water vapour pressure is lower where the temperature is lower. Although these patterns had been measured at 2 mm above the bark surface, they can be extrapolated to the small dimension of a corticolous arthropod, as demonstrated in Fig 26. Frequent saturation deficits in such crevices had indeed been demonstrated by LEWIS (1962), who conducted rough measurements with indicator paper.
In contrast, in the soils of temperate zones, cavities saturated with water vapour are accessible throughout the year in the litter layer (MILNE 1950, but see VERHOEF & SELM 1983) or at least directly below the litter zone. And these cavities are indeed often utilised by animals as refuge from desiccation (VANNIER 1970, METZ 1971, TAKEDA 1978, MacKAY et. al. 1987).
However, the point of desiccation inside the substrate bark was not reached by the trunks throughout the investigation period: at that point an increasing saturation deficit of ambient air would no longer result in an increasing evaporation, i.e. increasing difference of water vapour pressure between trunk and macroclimate (VANNIER 1970 for experimentally desiccated soils). This point would be very critical. Collembola, for example, die when their cuticula reaches this point (BLOCK et. al 1990), and their activity already ceases much earlier (VANNIER & VERDIER 1981). When soils are artificially driven up to this point, even the most tolerant oribatid species leave the substrate (VANNIER 1970).
4) Favourable humidity and heat (as ecological resources in the sense of MAGNUSON et. al. 1979) were seasonally antagonistic on trunks but not in the litter layer and the soil below. There, water is often even in oversupply during low temperatures (due to partial inundation of soil pores, VANNIER 1970).
5) For an optimal selection from the available microclimates a microarthropod would need to be aware of the important climatic gradients between trunk faces. This requires that the animal recognises the climate change on a scale of hours or of several decimetres separately from the much more frequent and small-scale climate changes within the microrelief. Moreover, the influences on thermic and hygric conditions were variable, whereas there was hardly a direct effect of more reliable constantly distributed factors, such as: (i) exposure of trunk faces to the main wind direction, (ii) exposure of bark ridges to wind, or (iii) the exposure of trunk faces to the main direction of rain and to the main run-off lines on the trunk.
4.1.3.2. Climatic advantages and possible adaptive strategies of arthropods:
1) Upheating of the trunk created a potential energy benefit (NICOLAI 1985, BÜCHS 1988). In the litter zone of soils, such upheating is largely prevented due to shading by the surrounding vegetation (direct comparisons between temperature curves on trunk and in litter: HAARLOV & PETERSEN 1952). But even there, heat supply accelerates the microarthropod population dynamics (VANNIER 1970, LUXTON 1981 a, b, v. STRAALEN 1985, v. STRAALEN & JOOSSE 1985, VEGTER 1987). Energetically adaptive distributions of animals should thus correspond to the relevant variables revealed by the respective multiple regression analysis.
2) The trunk surfaces were exposed to water vapour influx, even during sunny weather and daytime. This water vapour is probably available to the animals despite the decreased absolute water vapour pressure at the trunk because of the following effects: (a) the flux is directed towards relatively cool trunk boundary layers with a low saturation point. The saturation deficit therefore becomes especially low (zero, if condensation is the driving force of the water vapour influx). Moderately low saturation deficits of 89 Pa can still be temporally tolerated by the bark-dwelling Collembola species Orchesella cincta (calculated from VERHOEF & PRAST 1989). Bark-dwelling Psocoptera and Oribatei are even more tolerant (MADGE 1965, PRINZING & WIRTZ in press). And Psocoptera can suck water vapour from unsaturated air of 352 Pa saturation deficit (calculated from RUDOLPH 1982 for Cerobasis guestifalica). (b) Arthropods can also utilise water vapour that flows directly into the substrate by grazing on or mining in cryptogams (JENTSCH 1940 for Psocoptera, AGRELL 1941 and BAUER 1979 for Collembola, TRAVÉ 1963 for Oribatei). In air of only 80-90% relative humidity, lichen thalli can often reach a water content of 30-50 % of the thallus’ dry weight (HEATWOLE, 1966, roughly 50 % at 370 Pa saturation deficit). The air in the inner cavities or in the oribatids' mines of such moistened thalli is probably as saturated as in soils with an equally high water content (VANNIER 1970). (c) Even if the hygroscopic substrate cannot be grazed upon because it is too stiff or contains too many lichen acid crystals, this does not prevent some species from "sucking" the substrate's liquid water: Soil-dwelling Collembola of 200 and 300 mOsm haemolymph pressure can suck humidity from substrates covered by air of 6.1 and 13.3 Pa saturation deficit (under steady state conditions, calculated from EISENBEIS 1982 combined with data from ARLIAN & VESELICA 1979). Bark-dwelling Collembola should overcome even drier conditions due to their haemolymph pressures of 351 and 387 mOsm (Orche-sella cincta and Entomobrya nivalis, VERHOEF & WITTEVEN 1980). Most spiders can suck humidity, even from soils of only 12 % water content (FOELIX 1979). This corresponds roughly to steady state air humidities of ca. 187 Pa saturation deficit (calculated from VANNIER 1970 and SCHROEDER 1984, except for sandy, salty or compacted soils).
All above-mentioned saturation deficits tolerable for survival or even water vapour uptake were relatively more frequent during influxes of water vapour than otherwise (e. g. 13 Pa sat. def.: Chi² = 7.44, p < 0.01, df = 1, n = 443; 187 Pa sat. def.: Chi² = 6.53, p < 0.01; 387 Pa sat. def.: Chi² = 8.02, p < 0.01).
Adaptive distributional strategies should correspond to the patterns favouring either such an influx or favouring absolutely low saturation deficits (see multiple regression analysis in Tab. 1 and 2). Even during a slight efflux of water vapour from the trunk surface the animals could gain access to an influx by colonising crevices a few millimetres below the surface (Fig. 26). Fruticose (shrub-like) thalli exceed up to 2.5 cm above the bark and probably build up a separate system of microclimatic gradients starting from the outer border of each single thallus shrub (KERSHAW & FIELD 1975). Animals in such thalli could adapt their position within each thallus shrub.
3) Climatic zones with improved hygric or thermic conditions were dispersed regularly over each trunk. Especially trunk face zonations were relevant for heat and humidity conditions. Microrelief zonations, on the other hand, were especially persistent to wind turbulences and could enable the animals to compensate for a changing climate of the respective trunk face.
4) The absolute and relative supplies with water vapour and heat were not antagonistical with regard to the most important climatic variables. Also, during October and September, the seasonal antagonism between average temperature and humidity was not very strong.
5) Each of the possible thermic and hygric advantages of the trunk climate depended on effects on at least three different scales (macroclimate, trunk/ macroclimate interactions, trunk faces, microrelief zones). Thus, an animal could often simultaneously benefit from a thermic and a hygric advantage. The former could for instance be realised on a large scale or due to absolute conditions, the latter on a small scale or due to an influx of water vapour. The significance of such interac
tions between scales for the animals' abiotic living conditions has already been claimed by WILLMER (1982) and ANDREWATHA & BIRCH (1984). But to my knowledge it has never been described quantitatively for a natural system before (for an experimental system see BAUER 1979).
The following discussion will investigate, whether the colonisers’ distribution corresponds more to these advantages of trunk microclimate than to other possible benefits of trunk colonisation.
4.2. Use of climatic gradients by corticolous arthropods
4.2.1. Significance of climate use: Which benefits of trunk colonisation are
achieved?
4.2.1.1. Interpretability of the data
The verification of the different possible benefits of trunk colonisation requires the comparison of the climate with the distribution of species. Such comparisons can, with caution, be interpreted causally, because: (a) The distribution of a species is always influenced by the encountered climate, whatever the dominant proximate cause of the distribution is. Such proximate causes can include e. g. competition or predation. Yet, the animals are so sparsly distributed that they by far cannot occupy all the patches of the relevant microhabitat types within the relevant, short climatic periods (e.g. temperature zonations). This strongly limits the influence of inter- and intraspecific interactions on the general climate use of a species. After being caused by climatical effects, the distributional patterns could, however, be modified by predation or competition. Such effects are currently analysed. (b) Pseudocorrelations of climate with distributions of microhabitats (= food sources and microshelters) were prevented by the methodology. (c) The comparisons were not biased by a change in the microhabitat use (Fig. 19, pages 53-55): Species using similar microhabitats during certain climates often simultaneously changed very differently in their overall frequencies during these climates (which indicate climate use), e. g. Cerobasis guestifalica and Porcellio scaber during day and night; Cerobasis guestifalica and Orchesella cincta under different wind exposures. Correspondingly, species with different redistribution between microhabitats often changed similarly in their overall frequencies, e. g.: Orchesella cincta and Entomobrya nivalis and E. albocincta during cloudy and rainy weather; Porcellio scaber and either Carabodes labyrinthicus or Cerobasis guestifalica or Entomobrya albocincta on cooler and warmer microrelief zones; Orchesella cincta and Entomobrya nivalis on upheated and cooled trunks.
Before discussing the interaction of species’ distributions with different possible benefits, I will assess the reliability of the described diurnal dynamics and the significance of the seasonal dynamics:
Day-night-comparisons were not biased by a possibly lower optical searching efficiency at night. The evidence is: (1) Nocturnal decreases were not stronger in inconspicuous species (i.e. small, dark or slow): The oribatid Xenillus discrepans is darker than the encountered juvenile Carabodes but was nevertheless found more frequently during night. The oribatids Carabodes labyrinthicus and Xenillus discrepans are comparatively similar in size, speed and colour but were found in completely opposite diurnal distribution. Largest ("adult") Collembola are more conspicuous than the smaller "middle aged" ones, but nevertheless the latter were found to increase nocturnally. Temnostethus gracilis (Heteroptera) was hardly found at night, although it is especially easy to spot due to its larger size and its use of exposed crust microhabitats (Fig. 17, page 48). (2) Nocturnal decreases were not stronger in those microhabitats which were more difficult to survey: thalli of Evernia prunastri or horizontal crevices (Fig. 19, page 53).
The observed seasonal patterns might depend to a certain degree on dynamics of populations in adjacent litter layers. In this case, the seasonal patterns would be difficult to interpret with regard to benefits of trunk colonisation. For the following species this effect is very unlikely, because they are only very rarely found in litter but mostly colonise bark, rocks, walls, or cryptogams in dry or exposed habitats: Cerobasis guestifalica, Reuterella helvimaculata (JENTSCH 1940, GÜNTHER 1974), Bdella cf semiscuta (MICHOKA 1987), Temnostethus gracilis (WAGNER 1961, NICOLAI 1985), Xenillus discrepans (TRAVÉ 1963, WOAS pers. commun.), Entomobrya albocincta (CASSAGNAU 1965, BONNET et. al. 1975, PONGE 1993), Entelecara penicillata (WUNDERLICH 1982, NICOLAI 1985; REINKE & IRMLER 1994 for the investigated part of Germany). For the last three species’ distribution in the investigated part of Germany also: IRMLER, EMDE, pers. commun. and the database of "Forschungsstelle für Ökosystemforschung", Kiel. Other species are characteristic for and most frequent on tree trunks, walls, lichens on rocks etc. but nevertheless also found frequently and in lower dominance in forest litter: Carabodes labyrinthicus (quotations given in KLIMA 1954; KNÜLLE 1957, WUNDERLE 1992 a; IRMLER, EMDE pers. commun.) and Entomobrya nivalis (GISIN 1943, ELLIS 1974, BONNET et. al 1975, ALLMEN & ZETTEL 1982, PONGE 1993, IRMLER, EMDE pers. commun.). Populations of Entomobrya nivalis on trunks do not seem to have much exchange with populations in the litter, since in the latter a melanistic form is very often found, but it never occurs on the trunks (ELLIS 1974, PETERSEN pers. commun, pers. obs.). Only Orchesella cincta (GISIN 1943, VEGTER 1987, PONGE 1993) and Porcellio scaber (DEN BOER 1961, HARDING & SUTTON 1985) are frequent colonisers of litter.
Reproduction on the exposed trunks would make the seasonal dynamics additionally independent of dynamics of neighbouring populations. Such reproduction has already been demonstrated for most of the arthropod species considered: (i) for Xenillus discrepans, Carabodes labyrinthicus, Entomobrya albocincta, Cerobasis guestifalica, Reuterella helvimaculata, Bdella cf semiscuta by laboratory rearing of bark (collected in March 1995) from which all arthropods except the eggs had been removed by breaking it into small pieces, brushing and beating it out; (ii) for Entomobrya nivalis, Carabodes labyrinthicus and Entelecara penicillata by bark-emergence eclectors (BÜCHS 1988); and (iii) Cerobasis guestifalica and Reuterella helvimaculata by JENTSCH (1940) and SCHNEIDER (1955).
Qualitative observations in litter next to the investigated trees confirmed the mentioned habitat characterisations from literature. Moreover, during mid April 1996, 22 of the investigated trees were re-examined quantitatively by searching the bark and the surrounding litter for five minutes each, using a hand lens (10x magnification). The search revealed Entomobrya nivalis, E. albocincta and Carabodes labyrinthicus exclusively on the trunks (p < 0.01, Kolmogorov-Smirnov-test of goodness), while Orchesella cincta occurred almost exclusively in the litter (p < 0.01, K.-S.-test of goodness).
Thus, apart for Orchesella cincta and Porcellio scaber, substantial effects of litter populations on the seasonal dynamics on trunks are very unlikely due to a lack of dense litter populations and due to the ability to reproduce on the exposed trunks. Even for Porcellio scaber substantial effects are not very likely, either, since population dynamics in litter (MEINERTZ 1949, DEN BOER 1961) are very different from those recorded on the trunks. Also in Orchesella cincta dynamics of populations in the litter (VEGTER 1987, but VERHOEF & SELM 1983) are often different from those found on trunks. Even similar density fluctuations of Orchesella cincta in litter and on the trunks might be mutually independent: BÜCHS (1988) catches Collembola hatching on the trunks separately from vertically migrating animals. The seasonal dynamics of the "bark-born" animals, which can hardly depend on dynamics of litter populations, resemble those I encountered for Orchesella cincta and Entomobrya albocincta in the present investigation.
4.2.1.2. Microclimatic benefits
The possible microclimatic benefits are listed in the discussion of microclimatic results (4.1.3.2., page 76). Distributions of Collembola and also Porcellio scaber, Entelecara penicillata and juvenile Carabodes indeed corresponded much more to the expected distribution of thermic and hygric resource supplies than to any of the non-microclimatic benefits. Distributions of almost all species corresponded to the opportunities to choose between different climatic zones or to combine different abiotic resource supplies on different scales. Only the adult Carabodes labyrinthicus and Xenillus discrepans were distributed independently of all considered benefits (microclimatic and others). In a different environment, however, also Carabodes labyrinthicus can be positively correlated to trunk-upheating: during winter on forest tree trunks colonised by dense, strongly fluctuating populations (NICOLAI 1985).
The following paragraphs will summarise the encountered interactions with microclimatic benefits and demonstrate that equal benefits were often accessible to species of very different physiology and morphology (taken from the literature) and vice versa. Thus, insights from the presented distributional patterns in the field and from the published laboratory studies are different and cannot replace each other.
The distribution of Porcellio scaber and Entelecara penicillata strongly corresponded to heat-favouring patterns. This similarity is surprising when taking into account the differences in all remaining characters: The Isopod Porcellio scaber is large (up to two centimetres and more, GRUNER 1965/66), desiccates within hours in the laboratory (GUNN 1937, PRINZING & WIRTZ in press), mostly dwells in the litter (DEN BOER 1961), is bi- or trivoltine (VERHOEFF 1917, HEELEY 1941, WARBURG 1993), and grazes on algae and detritus (DEN BOER 1961, pers. obs.). In contrast, the spider Entelecara penicillata is small (1,5 mm, HEIMER & NENTWIG 1991), survives drought in the laboratory for more than a day (pers. observ.), is obligatory arboreal (NICOLAI 1985, WUNDERLICH 1982), univoltine (NICOLAI 1985) and predatory.
Conditions favouring heat were not or hardly reflected by distributions of Orchesella cincta, Entomobrya albocincta, the Psocoptera and the oribatids. In contrast, on seasonal scales heat accelerates the ontogenesis as well as the seasonal population dynamics of Collembola and oribatids in general (v. STRAALEN 1985, HIJII 1987, ASKIDIS & STAMOU 1991, STAMOU et. al. 1993) and of the Collembola Orchesella cincta and the Psocoptera Cerobasis guestifalica in particular (v. STRAALEN & JOOSSE 1985 and JENTSCH 1940). No species' distribution depended on low temperatures.
The distribution of Entomobrya nivalis and also of juvenile Carabodes corresponded to patterns favouring an upheating of the trunk. In contrast, Orchesella cincta, Entomobrya albocincta and Bdella cf semiscuta faced a cooling of the trunk. Apart from this, the Collembola (Entomobrya nivalis, E. albocincta, Orchesella cincta) are much more similar to each other than to either juv. Carabodes or Bdella cf semiscuta: These Collembola (1) use litter in similar macrohabitats (GISIN 1943, 1960, JOOSSE & VELTKAMP 1970, VEGTER 1985, PONGE 1993), (2) prefer similar abiotic conditions and are similarly tolerant to desiccation (JOOSSE & VELTKAMP 1970, MÜLLER-KRAENNER 1990, PRINZING & WIRTZ in press; no data for Entomobrya albocincta), (3) have similar surface-to-volume-ratios (for general effects see EISENBEIS 1989), (4) move much faster than the oribatids but much slower than Bdella cf semiscuta (pers. obs.), (5) graze on layers of algae or phycobiots, whereas juvenile Carabodes largely mine in lichen mycobiots (PRINZING & WIRTZ in press, and 4.3.1.) and Bdella cf semiscuta is predatory (EVANS et. al. 1961, pers. obs.), (6) mature faster than oribatids (SOLHOY 1975 for Carabodes labyrinthicus, JOOSSE et al. 1973 for Orchesella cincta and other Collembola), (7) belong to almost the same ecological life forms among the Collem-bola according to many morphological traits defined by v. TÖRNE (1953).
High temperatures at or upheating of tree trunks are also made responsible for the winter activity of many corticolous spiders, Collembola, Acari, Chilopoda, Diptera, Hymenoptera, Coleoptera, Thysanoptera and Aphidina, while conspecific animals or related species in the soil are mostly dormant during winter (NICOLAI 1985, BÜCHS 1988).
The distribution of Orchesella cincta and also Entomobrya albocincta strongly corresponded to patterns favouring low saturation deficits. The effect of Entelecara penicillata's distributions was opposite. This, indeed, fits well to the generally high drought sensitivity in Collembola compared to spiders. However, the similarly tolerant oribatids, Psocoptera or bugs (MADGE 1965, VANNIER 1970, RUDOLPH 1982, PRINZING & WIRTZ in press, pers. obs.) did not correspond to the conditions that determined saturation deficit.
The distribution of Entomobrya nivalis strongly corresponded to patterns favouring influxes of water vapour. Even the position along the climatic profile perpendicular to the trunk surface corresponded to the probable influxes of water vapour (according to Fig. 26): an increasing use of the trunk surface during a slight cooling, and of deeper positions during a stronger cooling. Distributions in the other Collembola were much less clear-cut. Entomobrya nivalis is indeed the Collembola with the highest osmolarity of the haemolymph measured so far (including Orchesella cincta, VERHOEF & WITTEVEN 1980, EISENBEIS 1982). Entomobrya nivalis is correspondingly especially capable of utilising inflowing water vapour either (i) by absorbing the liquid water that accumulates at the bark surface through the ventral tube despite the binding forces of the substrate (description see 4.1.2.: humidity profile at the bark surface, page 73) and against the osmotic pressure of dissolved ions (EISENBEIS 1982); or (ii) by cuticular absorption of water vapour from saturated air (after dehydration, MAIS 1970, VERHOEF & WITTEVEN 1980).
Porcellio scaber and Entelecara penicillata were tightly coupled to water vapour effluxes. In Porcellio scaber this could also be compared to the position along the climatic profile perpendicular to the trunk surface (Fig. 26). Also on this scale, Porcellio scaber was exposed to an efflux of water vapour. This would in fact enable Porcellio scaber to reduce the excess of water gained during the frequent diurnal stays in the moist litter layer (DEN BOER 1961). In contrast, in Entomobrya nivalis, which uses conditions of influxes, there was much less evidence for such a diurnal shuttling towards moister layers. Correspondingly, this Collembola did not need to use the trunk as a "drainage" for body water. Only in a habitat with an adjacent, large water reservoir (stone heaps on soil) even a Collembola species (Orchesella villosa) uses zones of water vapour effluxes (inferred from EISENBEIS 1983).
Simultaneous supplies of different abiotic resources on different scales could be used by Collembola, Porcellio scaber and Temnostethus gracilis. The equivalence of a humidity supply from different scales (moist bark or moist ambient air) has already been demonstrated experimentally for corticolous Collembola by BAUER (1979).
Distributions of all species, except of the adult Carabodes labyrinthicus, corresponded to the opportunity to choose between microclimatic zones (including the wind exposures). Among the hygric or thermic zonations, trunk faces were always (and sometimes solely) relevant. This corresponds to their large effect on the microclimate (Tab. 1, 2 pages 23, 27), despite the difficulties in perception of such large-scale, uncontinuous patterns (see 4.1.3., page 75). Microrelief zones were, however, selected to compensate for a cooling of the trunk face or, in Orchesella cincta, to intensify its effects on the animals. Orchesella cincta can also intensify the effect of coldness on a seasonal scale by physiological mechanisms (v. d. WOUDE & VERHOEF 1988): an interruption of the cold diapause requires the highest temperatures during the coldest season. Such intensifications on large scales might help to avoid the effects of the frequent climatic disturbances on smaller scales (v. d. WOUDE & VERHOEF 1988).
Thermic and hygric zonations were primarily reflected by the distributions of Collembola, not of Psocoptera or oribatids. This is probably due to the fact that (1) the oribatids can only perceive humidity of a substrate that surrounds the animal (RIHA 1951) - which is not the case on solid bark. Indeed, the humidity receptors are situated on the forelegs (MADGE 1964), without direct contact to the substrate surface, i. e. the layer of the most intense climatic gradients. On solid surfaces, oribatids react correspondingly very slowly to humidity gradients (WALLWORK 1960, MADGE 1964) and without any directed movements (MADGE 1964). The Collembola and Psocoptera have a much better developed humidity perception at the substrate surface (by ventral tubes and probably also the empodium, and by the hygroscopic labium, respectively) and also eyesight (STREBEL 1932, BLADONELL 1951, SCHALLER 1969, VANNIER 1970, RUDOLPH 1982, EISENBEIS 1983, LEINAAS & FJELLBERG 1985). (2) The Psocoptera are metabolically least capable of utilising zones of increased temperature: Q10 values (for respiration) in arboreal Psocoptera (including Cerobasis guestifalica) only range from 1.3 to 1.5, compared to 1.7 to 11.3 in Entomobrya nivalis (TURNER 1983), 2.5 to 8.0 in Orchesella cincta (v. d. WOUDE & JOOSSE 1988) and 2.6 to 4.0 in oribatids from central Europe (BERTHET 1964 b, WEBB 1969). In contrast, different migration capabilities can not be very important for the different use of climatic zonations, since even Carabodes labyrinthicus can walk at a speed of 1.8 cm/min (WUNDERLE 1992 a), and BERTHET (1964 a) found that Xenillus tegeocranus can migrate for up to 40 cm air line per day, apparently without being attracted by an environmental gradient (each of the animals tested took a different direction).
In contrast to the other benefits considered later on, those due to the microclimate are always realised on certain parts of a trunk - just like the general presence of cryptogams as potential food. The significance of such continuous benefits would explain: (1) the continuous colonisation of bark, often including reproduction (for oribatids: TRAVÉ 1963, ANDRÉ 1975, NICOLAI 1985, BÜCHS 1988, WUNDERLE 1992a; for Collembola: BÜCHS 1988, MÜLLER-KRAENNER 1990; for Psocoptera: JENTSCH 1940, GÜNTHER 1974, NICOLAI 1985, 1986, PRINZING & WIRTZ in press); (2) phenologies which are independent of immigration from the litter (BÜCHS 1988); and (3) the animals' accurate and adaptive differentiation between typical bark microhabitats (see 4.3.3., page 98, ANDRÉ 1975, 1976, PRINZING & WIRTZ in press). Populations of many arthropod species on tree trunks could thus use the food sources on bark largely autarkically from the habitat soil. Thus, the bark was more than a transitional habitat (as argued by DELAMARE-DEBOUTEVILLE 1951) despite of its depoverated fauna. Instead, this species-poor fauna might be due to the extreme microclimates or the small habitat volume.
4.2.1.3. Connection between soil and crown
The expected interaction of trunk colonisation with the crown´s vegetation period was only found with respect to five speciec. But even in these species the exact density or the age class compositions did not correspond to the crowns´ phenology at all. Moreover, at least two of these species (Cerobasis guestifalica and Reuterella helvimaculata) are completely absent from the soil- or herb-layer (GÜNTHER 1974), which makes the trunk useless as a soil-crown-migration route. Also, the rather precise increase of Collembola densities (Entomobrya albocincta, Orchesella cincta) at the end of the vegetation period were not caused by a soil-crown migration, as discussed in 4.2.1.1. Also in spring (studied by NICOLAI 1985, 1986, BÜCHS 1988 and WUNDERLE 1992 a), the crown's vegetation period does not correspond to the abundances of corticolous Entomobryids, Carabodes labyrinthicus, Cerobasis guestifalica or Entelecara penicillata, nor to the migratory activity of Carabodes labyrinthicus. Soil-crown-migrations were therfore probably not the primary cause for trunk colonisation in most individuals found on the bark. This might explain the relatively stable vertical zonation of faunas on high forest trees (NIEDBALA 1969, BRAUN 1992, WUNDERLE 1992 a, SIMON pers. commun.). Ontogenetic redistributions of microarthropods between litter or trunk base and crown (CHRISTENSEN 1980, ALLMEN & ZETTEL 1982, WUNDERLE 1992a) reflect probably a gradual phenomen, not an obligatory change between strata.
4.2.1.4. Access to food sources due to moist macroclimates
Collembola are considered to use tree trunks as a food source - provided that the macroclimate is sufficiently humid (MEYER 1957, BOWDEN et. al. 1976, BAUER 1979). In the present investigation, all species except Cerobasis guestifalica, juvenile Carabodes and Temnostethus gracilis, corresponded to either moist weather, moist daytimes, or moist and warm seasons. But only in three cases (Xenillus discrepans, Orchesella cincta and, confirming CLOUDSLEY-THOMPSON 1973, Porcellio scaber) more than one of these shelters was relevant. In contrast, middle-aged Collembola corresponded unequivocally to moist daytimes and weather conditions - independently of the different seasons when the animals occurred (Fig. 15, page 43). This pattern could be explained as follows: (1) The proportion of middle-aged Collembola increased during macroclimatic shelter, because many of these animals are fertile males, which need moisture for a maximal reproductive success (JOOSSE, BRUGMANN & VELD 1973, MERTENS & BLANQUAERT 1980). In contrast, the oldest, largest animals are mostly fertile females, which reproduce most during heat. Increasing migratory activity in middle-aged and adult Entomobrya nivalis is already known from alpine spruce trunks (ALLMEN & ZETTEL 1983). (2) The proportion of adults increased during the day and sunny weather because they prefer highest temperatures (MÜLLER-KRAENNER 1990) and are probably more tolerant to drought due to their smaller surface-to-volume ratio (general effects in EISENBEIS 1989). (3) The proportion of youngest Collembola increased during the day and during sunny or cloudy weather because hatching of Collembola is accelerated by heat (v. STRAALEN & JOOSSE 1985; reproduction of Entomobryids on trunks is demonstrated by BÜCHS 1988 and MÜLLER-KRAENNER 1990). (4) The proportion of youngest Collembola remained high during the day and sunny weather because they were able to avoid drought in two ways without leaving the trunk: (4a) They sheltered in bark crevices (Entomobrya nivalis; in Orchesella cincta juveniles at least used crevices more than the middle-aged one did; Fig. 18, page 51). And indeed, on trunks that do not provide crevices as shelter for juveniles, most Collembola have to leave the trunk during the day or sunny weather (BOWDEN et. al. 1976 and BAUER 1979 who investigated wooden tripods and young beeches, respectively). (4b) The juvenile Collembola used moist and warm seasons to develop rapidly into the more tolerant middle-aged stage (see steep peak of juvenile Entomobrya albocincta in September and October in Fig. 15 (page 43) and STAMOU et. al. 1993 for similar developments in several other Collembola species).
In summary, the investigated fissured, lichen covered trunks were accessible to most animals without moist macroclimates. An important purpose of this colonisation was certainly the supply of cryptogam food which correspondingly was used very selectively (see 4.3.1.). This, indeed, confirms a part of the hypothesis of BAUER (1979) and MAYER (1957) and rejects VEGTER's (1983) speculative doubts about the significance of such food sources.
4.2.1.5. Shelter from soil soaking
Increased CO2 pressures and partial cover by water films in soaked soil and litter are responsible for trunk colonisation by Collembola according to FUNKE (1979) and VEGTER (1983). However, BAUER (1979) demonstrated in detail, that it is the environmental humidity above the litter which determines the upward migration of litter-dwelling Collembola on young, smooth beech trunks during rain. In the present study, on much more structured tree trunks, densities never increased more than 30 % during rain. The immigrants were apparently mostly middle-aged animals, which should not be more (but possibly less) sensitive to soaking of the soil / litter than other age-classes (see hypothesis in 3.2.4., page 44). Exceptions are: (1) Cerobasis guestifalica, which does not dwell in the litter, however, and (2) the tendencial increase of both middle-aged and adult animals in Orchesella cincta.
Moreover, most species' partial immigration during rain was well explicable as a use of increased environmental moisture, corresponding to the distributions on other scales. Nevertheless, the bases of trunks might be a significant refuge during rain. The upper trunk regions are only relevant during extreme floods (GAUER 1995 for amazonian forests).
4.2.1.6. Access to wind dispersal
The opportunity for wind dispersal corresponded to an increase in densities on at most a single scale. This is no reliable evidence because it could equally be explained by changes in the structure of microhabitats: Evernia prunastri's growth form is more dense and shrubby on wind-exposed sites (ZIMMER 1994, 3.4.3. page 66), crevices on wind-exposed bark ridges are mostly deeper than those in the bark valleys, while crusts of Lepraria incana are often thinner (pers. obs. during scraping off the Lepraria incana plots). The trunks might be a significant jumping-off point only during rare mass migrations of Collembola or for dispersal between isolated soils situated in the crowns of rainforest trees (FARROW & GREENSLADE 1992 or BLACKITH & DISNEY 1988).
4.2.2. Prerequisites: Does the climate use depend on r/K/A life strategy traits (taken from literature)?6)
Climate use is a part of a life strategy: In tendency, animals either (a) stay at one site where they find continuously favourable conditions ("K-strategy", unrealistic for arthropods on exposed trunks with their variable climate), or (b) change between unpredictable sites of momentary favourable conditions ("r-strategy"), or (c) stay at one site where they overcome the periods of unfavourableness ("A-strategy") (GREENSLADE 1983, SOUTHWOOD 1988).
Species that did frequently redistribute with changing microclimates (Collembola, Porcellio scaber) are expected to show "r-selected" traits (summarised in GREENSLADE 1983 and SOUTHWOOD 1988), many of which are indeed realised: (1) the migration is fast (compared to oribatids), (2) the maturation is either early at approximately 50 % of the maximum body size as in the Collembola, or the largest stage lives a long time and reproduces iteroparously as in Porcellio scaber (LINDEMANN 1950, JOOSSE & VELTKAMP 1970 for Orchesella cincta, MÜLLER-KRAENNER 1990 for Entomobrya corticalis Nicolet, VERHOEFF 1917 for Porcellio scaber), (3) the monthly fluctuations of population densities were larger than in other species (Fig. 12 b, page 38), (4) the fecundity is high in the Collembola with 60 - 100 eggs/female (LINDEMANN 1950, JOOSSE & VELTKAMP 1970) and the size of eggs is small (0.25 mm in diameter, JANSSEN & JOOSSE 1987; MÜLLER-KRAENNER 1992) and (5) the geographic distribution is wide-spread (GISIN 1960, HARDING & SUTTON 1985). Some other traits of the these Collembola and of Porcellio scaber, however, fulfil expectations for "K-strategy": (i) a low degree or complete lack of parthenogenesis (LINDEMANN 1950, WARBURG 1993), (ii) a low capacity for dormancy in the Entomobrya species, occuring throughout the year (Fig 12 b, BÜCHS 1988, MÜLLER-KRAENNER 1990), (iii) a moderate life span of > 10 months, as compared to the short-lived Psocoptera and the long-lived oribatids (MERTENS et. al. 1982, MEINERTZ 1949). Eventually, an "A-strategy" would be expected in Porcellio scaber with regard to the high behavioural investment by guarding the 50 to 60 eggs (HEELEY 1941, HEROLD 1960), but not regarding the comparatively low abiotic tolerances of Porcellio scaber (GUNN 1937, PRINZING & WIRTZ in press).
Species that had a more constant distribution (oribatids, Psocoptera) are expected to show "A-selected" traits (GREENSLADE 1983, SOUTHWOOD 1988). These are indeed largely realised in the oribatids but not in the Psocoptera: Oribatids (1) are slower than the other species, (2) live a long time: ca. 2 years in Carabodes labyrinthicus, (WUNDERLE 1991, 1992 a) and > 1 year in Xenillus tegeocranus, closely related to X. discrepans, (LUXTON 1981 a, b), (3) do not mature till the end of their lives (WUNDERLE 1992 a for Carabodes labyrinthicus), (4) are most tolerant to abiotic stress (MADGE 1965, PRINZING & WIRTZ in press), (5) produce few and large eggs (WUNDERLE 1992 a for Carabodes labyrinthicus, RIHA 1951 for oribatids in general), (6) fluctuate only moderately in their seasonal densities (Fig. 12 b, page 38). However, oribatids correspond more to a "K-strategy" with regard to the low degree of dormancy (WUNDERLE 1991, 1992 a, LUXTON 1981 a, b) and of parthenogenesis (according to sex ratios in SOLHOY 1975 and LUXTON 1981 a). The wide geographic range of these oribatids fits to an "r-strategy" (Xenillus discrepans also occurs in southern France, TRAVÉ 1963, Carabodes labyrinthicus occurs throughout Europe and even in north-American epiphytic lichens, REEVES 1988). In the Psocoptera, expectations for an "A-strategy" are only fulfilled by (i) the high/moderate tendency for parthenogenesis in Cerobasis guestifalica/Reuterella helvimaculata (JENTSCH 1940, SCHNEIDER 1955) and (ii) the high abiotic tolerance in Cerobasis guestifalica (RUDOLPH 1982, PRINZING & WIRTZ in press). Probably, Reuterella helvimaculata is less drought tolerant, since it is generally restricted to more mesic macrohabitats (GÜNTHER 1974). The Psocoptera appear to be "K-selected" with regard to the continuously changing (not fluctuating) seasonal abundances - but not regarding the high dormancy capacity and the moderate number and size of eggs (Cerobasis guestifalica lays ca. 80 eggs/female, each of > 20% of adult body length, JENTSCH 1940; no data available for Reuterella helvimaculata). The majority of the Psocoptera's traits, however, corresponds to the "r-selection" scheme: (1) a high mobility compared to oribatids, (2) a short life span of less than half a year (JENTSCH 1940, SCHNEIDER 1955, GÜNTHER 1974), (3) an iteroparous adulthood in Cerobasis guestifalica and also in Reuterella helvimaculata (JENTSCH 1940, SCHNEIDER 1955), (4) a wide-spread geographic distribution (GÜNTHER 1974). And in fact, physiologically the Psocopteras' climate use might indeed be "r-selected": The animals use the partly unpredictable periods of moist air opportunistically for absorption of a water reservoir (RUDOLPH 1982). Within a few hours at 85% rel. air humidity Cerobasis guestifalica can absorb enough water to overcome a whole (mid-European) summer day (RUDOLPH 1982). This permits the animals to maintain a high degree of physiological homoeostatis - an effect equivalent to a permanent search for favourable sites within the environment as it is characteristic for an "r-strategy".
In summary, the habitat templet (harshness, predictability) that a species experienced due to its redistribution corresponded roughly to a mosaic of "r-" or "A-selected" expressions of other traits (according to the schemes in GREENSLADE 1983 and SOUTHWOOD 1988). Exception: Species which change more opportunistically in their water vapour absorption than in their distribution.
4.3. Use of discrete microhabitats (cryptogam species, crevice types) by corticolous arthropods
The climatic gradients investigated up to now could only be utilised by the animals if they had enough food and climatic microshelter (e. g. from direct sun exposure) at any position along such gradients. The following discussion analyses, whether the animals managed to improve the food and microshelter supply actively: by their distribution within the extremely variable mosaic of discrete microhabitats, despite the strong climatic exposure and restricted palatability of the cryptogams. Besides these effects of microhabitat use, its true reasons and the prerequisites are also discussed. Due to the applied methodology, the discussion is not biased by the changing frequency of certain microhabitat types along some climatic gradients.
4.3.1. Significance of microhabitat use for the supply of food and climatic microshelter
Collembola: Orchesella cincta, Entomobrya nivalis, E. albocincta.
F o o d s o u r c e s are algae (JOOSSE 1975, pers. observ.) and phycobiots of Lepraria incana (pers. field observ. and studies of gut contents7) ) and of Evernia prunastri (PRINZING & WIRTZ in press). Among these food sources, only Evernia prunastri was found to be suitable during drought as well as heat or chill (except for Entomobrya albocincta during extreme temperatures). This confirmed laboratory experiments according to which grazers can be protected from desiccation in Evernia prunastri thalli - even they´re when dry (PRINZING & WIRTZ in press). Evernia prunastri was found to be equally suitable, when shelter was superfluous (except in wind-sheltered bark valleys during mesic climates). The crusts of Lepraria incana, in contrast, were mainly suitable when sheltered from upheating and thermic extremes (Orchesella cincta, Entomobrya nivalis), from drought (Entomobrya nivalis) and also from wind turbulences (especially Entomobrya nivalis). Also the algal crusts were suitable only when protected from radiation (Orchesella cincta, Entomobrya nivalis) and from wind turbulences (Orchesella cincta, Entomobrya nivalis, E. albocincta).
Only Orchesella cincta used algae in similar large densities as it used Lepraria incana. It is probably the only one of the three Collembola species that can break up the algal crusts when they are stiff and dry, and feed on them - just like it is the only one of the Collembola species which can break up the stiff cortex of Evernia prunastri (PRINZING & WIRTZ in press). The occasional presence of such algae in horizontal crevices could thus have provided sufficient food for Orchesella cincta and caused the crevices´ comparitively continuous colonisation by this species (according to the discriminant analysis).
Entomobrya albocincta is morphologically similar to Orchesella cincta but smaller. These animals are thus less capable of grazing upon stiff substrates, which even larger species can only break off by pushing and pulling with the whole body (PRINZING & WIRTZ in press on Evernia prunastri cortex). In Entomobrya albocincta, the stiff algal crusts were indeed overproportionally used by the "adults", i. e. the largest animals. In contrast, Lepraria incana crusts, which are much softer than algae, even when dry, were used equally also by small animals. In the similarly small Entomobrya nivalis, the soft Lepraria incana was even used overproportionally by the smallest animals.
Crusts of Pertusaria albescens were not used at all, even though they were mostly as soft (and hydrophobic) as Lepraria incana. However, Pertusaria lichens have different lichen acids (CULBERSON 1979), which in general can effect grazing intensity by animals (STAHL 1904, RUNDEL 1978, LAWREY 1980, 1983, REUTIMANN & SCHEIDEGGER 1987). Moreover, crusts of Lecanora expallens (and Lecanora conizaeoides, investigated additionally in 42 plots) were used less than the algal crusts, despite similar structure and stiffness. Again, this corresponds to the distribution of lichen acids. Lecanora and Pertusaria are both comparatively rich in phenolic carboxylic acid derivates (CULBERSON 1979).
Entomobryids also graze upon fungus mycelia and faeces (SCHALLER 1950, BERNARDI & PARISI 1968, WOLTERS 1985, PONGE et. al. 1993) as they occur frequently in vertical crevices (pers. observ.). However, this was apparently not a sufficient food source because the vertical crevices were largely abandonned whenever they were superfluous as shelter.
S h e l t e r s: The well protected vertical crevices were increasingly used during h e a t and d r o u g h t stress and, in Entomobrya nivalis, by the more drought-susceptible juveniles (which also prefer lower temperatures, MÜLLER-KRAENNER 1990). Horizontal crevices, in contrast, would indeed only provide little protection from heat and desiccation (measurements by LEWIS 1962) and were then apparently avoided by Entomobrya nivalis and E. albocincta. During c h i l l (or relative cooling), however, Entomobrya nivalis and E. albocincta were sheltered in horizontal crevices. Indeed, these crevices are free of hoarfrost or snow, which can otherwise cover the trunk's surface and make food inaccessible there. This is confirmed by occasional winter observations by AGRELL (1941) and ALLMEN & ZETTEL (1982). The vertical crevices were less suitable as chill-shelter, possibly because they are more narrow in winter (pers. observ. when breaking up the crevices). This might be due to the slow growth of trees in winter combined with the successive scaling off of older, lager crevices. The lack of palatable, soft Lepraria incana as food for Entomobrya nivalis and E. albocincta in both types of crevices might even increase the crevices´ function as climatic microshelter: starvation reduces the number of nuclei for ice formation and thus the risk of freezing (BALE & PULIN 1991 for starve Collembola in crevices of a wall). And starvation can also reduce the metabolism and thereby the rate of transpiration and desiccation (VERHOEF & LI 1983 for the closely related Orchesella cincta).
The considered Collembola species get trapped in a w a t e r f i l m and drown very easily (PRINZING & WIRTZ in press). Algae and especially horizontal crevices, which easily get covered/filled up with water, were thus not suitable when exposed to precipitation. This contrasted to the hydrophobic crusts of Lepraria incana and to protected vertical crevices and the protected cavities between branches of Evernia prunastri.
In consequence, the observed patterns of average Collembolan densities on different microhabitats could be completely explained by the availability of adequate food and microshelter: the largest average densities in Evernia prunastri (food and all kinds of shelter), less in horizontal crevices (chill-shelter and, for Orchesella cincta, sparse algal food) and in vertical crevices (heat and drought shelter), similar, moderate densities on the adequate food source and waterfilm shelter Lepraria incana and, for Orchesella cincta, on the food source algae. Only in Entomobrya albocincta, were the average densities in vertical crevices relatively higher, which probably reflected a higher preference for the fungal hyphae in these crevices or a stronger need for shelter. Such need can be inferred from: (a) the geographic distribution: moist (atlantic) regions such as southern Scandinavia and western Europe (GISIN 1960), including Belgium (ANDRÉ 1976, PONGE 1993), absence in montane, central Europe (GISIN 1943, MÜLLER-KRAENNER 1990) and (b) the use of slightly moister mesohabitats according to PONGE (1993).
Psocoptera
Cerobasis guestifalica's f o o d s o u r c e s are algae (JENTSCH 1940) and phycobiots of Evernia prunastri (including the cortex, PRINZING & WIRTZ in press) and also phycobiots/algal epibiots of Lepraria incana (field observ., in guts of all specimens from bark crevices I found primarily Lepraria phycobiots, as described in footnote 7, page 91). The food source Evernia prunastri was used even during harsh climates, whereas algae and Lepraria incana had to be sheltered from wind for colonisation and algae had to be additionally sheltered from waterfilm.
S h e l t e r s: S u n n y weather was often withstood in horizontal crevices. This resembled the oribatid Carabodes labyrinthicus but contrasted to the less drought-tolerant animals: Collembola and Porcellio scaber (for tolerances see MADGE 1965 for C. labyrinthicus, VERHOEF & WITTEVEN 1980 for Entomobrya nivalis and Orchesella cincta, RUDOLPH 1982 for Cerobasis guestifalica, and PRINZING & WIRTZ in press). Only hatchlings of Cerobasis guestifalica used the more sheltered, vertical crevices overproportionally, corresponding to the generally higher demand of juveniles for moist shelter due to the larger surface to volume ratio (for general effects see EISENBEIS 1989). Indeed, the relative densities of Cerobasis guestifalica in vertical crevices increased exactly when hatchlings were most frequent: during the day and rainy weather (Fig. 16, page 45). From these vertical crevices, an ontogenetic succession started, passing through horizontal crevices and leading onto parts of the bark surface and into the attached thalli of Evernia prunastri.
The c h i l l -shelter function of horizontal crevices (mentioned for Entomobrya nivalis and E. albocincta) was required much less by a summer-to-autumn-species like Cerobasis guestifalica. Chill-shelter might nevertheless have been responsible for the increasing use of crevices on cooled trunk faces. The resistance of Cerobasis guestifalica to w a t e r f i l m s is not much larger than in the Collembola (PRINZING & WIRTZ in press) and limited the use of microhabitats, namely algae, which are otherwise preferred during rainy weather. Shelter from waterfilm might have been due to cavities between Evernia prunastri branches and the hydrophobe Lepraria incana crusts.
In summary, the sequence of average densities on microhabitats again corresponded to their probable functions: Evernia prunastri provided food and mostly shelter, vertical crevices provided shelter for the numerous hatchlings, horizontal crevices provided desiccation shelter and occasionally algae as food source (such as in Orchesella cincta), Lepraria incana provided only shelter from waterfilm and food, algae provided food.
Reuterella helvimaculata can graze like Cerobasis guestifalica (BROADHEAD 1958 a; for Evernia prunastri as food: LAUNDON 1971, pers. obs). Largest densities in Evernia prunastri probably reflected the same effects of food and shelter as in Cerobasis guestifalica. In contrast, Reuterella helvimaculata might have been able to use unsheltered food sources like Lepraria incana relatively more frequently, because less climatic microshelter was needed: (1) the species was largely nocturnal (Fig. 12 b, page 38). (2) The males, which were over-represented on Lepraria incana, are winged, and thus could easily retreat into other, climatically more sheltered macrohabitats (e. g. the crown).
Oribatids
Adult Carabodes labyrinthicus: F o o d s o u r c e s are phycobiots and cortex of Evernia prunastri (PRINZING & WIRTZ in press, BELLIDO 1979 for Carabodes willmanni on similar lichen thalli), the mycobiot layer of the soft, thick specimens of Lepraria incana and Pertusaria albescens crusts, often used for digging mines (pers. obs., further references for lichenophagy see SEYD & SEAWARD 1984) and detritus penetrated by hyphae (WUNDERLE 1992 a, pers. obs.) as it accumulates in vertical crevices. Carabodes labyrinthicus found s h e l t e r in Evernia prunastri (from heat, drought and wind turbulence), and also in horizontal crevices (from heat) and in vertical crevices (from sunny weather). But even then a large number of animals was sheltered in the mines dug into Lepraria incana. Again, the combination of lichen-food and shelter (i. e. mining substrate or cavities ) corresponded to the highest average densities on microhabitat types, as found on Lepraria incana or in Evernia prunastri. Indeed, the only other microhabitat which can be mined, Pertusaria albescens, was the type of crust used second most. The preference for Lepraria incana compared to the similar Pertusaria albescens, and for algae compared to the similar Lecanora expallens (and the 42 sampled plots of Lecanora conizaeoides) corresponded to thallus chemistry, as mentioned already for the Collembola.
The juvenile Carabodes and adults of Carabodes labyrinthicus used similar trunk climates (Fig. 12, pages 37, 38) and both dug mines into crusts of Lepraria incana. Juveniles also dig into Evernia prunastri (PRINZING & WIRTZ in press, BELLIDO 1979: similar behaviour in juvenile C. willmanni). The pattern of microhabitat colonisation strongly resembled that of adult Carabodes labyrinthicus, and was probably explicable by similar mechanisms.
The oribatid Xenillus discrepans did not dig mines (as the above oribatids did) and had no recognisable difficulties in breaking up the stiff algal crusts (as Entomobrya species probably have). Correspondingly, there was no need to prefer a soft crust like Lepraria incana. Shelter from desiccation was hardly provided by the most frequently used microhabitats, but was ensured by the animals' largely nocturnal colonisation of the exposed trunk (Fig. 12 b, page 38).
The Isopod Porcellio scaber can adapt to many f o o d s o u r c e s , including detritus and algae (DEN BOER 1961). Grazing on Evernia prunastri was not observed (PRINZING & WIRTZ, in press; pers. observ.). During mesic conditions, densities increased in microhabitats that provided a slight epibiotic algae cover (as potential food) - especially if also some climate shelter was provided in addition: in Evernia prunastri and horizontal crevices. The bark detritus which accumulated in vertical crevices did not seem to be sufficient as food, since most animals only stayed there when s h e l t e r from r a d i a t i o n was needed. Nevertheless, this primary shelter function of vertical crevices would have been a sufficient explanation for the comparatively largest average densities found here, because Porcellio scaber is the most drought sensitive among the considered species (GUNN 1937, PRINZING & WIRTZ in press). Porcellio scaber can avoid w a t e r f i l m s (PRINZING & WIRTZ in press). Correspondingly, hydrophobic thalli of Lepraria incana were not used more when water films occurred.
Predators
Bdella cf semiscuta (Trombidiformes, Acari): The f o o d s o u r c e s observed to be used on the investigated trunks were small isotomid Collembola (pers. obs.). These animals mined mostly in the soft bark adjacent to vertical crevices (where they could not be sampled in the present study) and sometimes colonise Evernia prunastri (PRINZING & WIRTZ in press). Both microhabitat types also provided climate s h e l t e r and were indeed colonised most densely by Bdella cf semiscuta. Entomobryids, Psocoptera and oribatids might be further prey items according to observations on related bdellid species (BROADHEAD 1958 b, TURNER 1984, STAMOU & ASKIDIS 1992, SWIFT pers. commun.). But they were not observed as prey during the present study despite that they were much more common than the Isotomids. In any case, these potential prey items would also be most frequent in Bdella cf semiscuta's favourite microhabitats Evernia prunastri, vertical and horizontal crevices. The latter were also used by this mite for pauses after the characteristic rapid crossing of one or few decimetres of exposed bark surface. Eventually, the moderately large densities on Lepraria incana corresponded to the preferred e g g l a y i n g s i t e (providing shelter from waterfilm).
Temnostethus gracilis, Loricula elegantula (Heteroptera) and Tachypezia nubila (Diptera): F o o d s o u r c e s are grazers as large as Psocoptera (DELAMARE-DEBOUTEVILLE 1951, BROADHEAD 1958 b, PÉRICART 1972, CHVALA 1975, RICHARDSON 1975, NICOLAI 1985, pers. obs.). Nevertheless, these predators cannot or badly hunt where most of such potential prey animals settled: in Evernia prunastri or in crevices. Here, there is no space or light for sight-and-attack hunting (Tachypezia nubila and bugs) and no soft substrate for poking (bugs). The two bug species, thus, were especially frequent on either of the two soft crust lichens: Lepraria incana or Pertusaria albescens. This was a very differentiated niche segregation. S h e l t e r : The animals were not sheltered where they were observed hunting. Instead, the bugs, especially the often juvenile Loricula elegantula, equally colonised certain cavity microhabitats. The empidid Tachypezia nubila was absent in such cavities. Instead, it was climatically well protected due to (i) its largely nocturnal colonisation of the exposed trunks (Chi² = 7.4, p < 0.001, n = 34), (ii) its ability to leave the exposed trunk quickly by rapid migration and (iii) probably by its strong sclerotisation.
The spiders Lathys humilis and Entelecara penicillata were the only predators that colonised primarily Evernia prunastri. This corresponds to the
s h e l t e r e d microclimate, to the high density of potential f o o d s o u r c e s (prey) and to the spiders' ability to catch prey within small cavities and on stiff substrates. The prey might even be less capable of avoiding predation in Evernia pru-nastri, because the three dimensional microhabitat structure might complicate the perception of spider threads by prey animals (by palpating the surrounding with legs that bear long setae or with the antennae; PRINZING 1992, pers. obs).
Entelecara penicillata's larger densities in vertical than in horizontal crevices corresponded to the distribution of most potential prey and to the most effective climatic microshelter. Lathys humilis' more intense use of horizontal crevices corresponded to the juveniles' distribution: These animals might have needed horizontal crevices as a network of sheltered i m m i g r a t i o n r o u t e s onto the trunks (Lathys humilis does not breed on the trunks as Entelecara penicillata does, BÜCHS 1988).
4.3.2. Alternative explanations for microhabitat use
In addition to or instead of the discussed factors, the microhabitat use might have been determined by e.g. natural enemies, competitors or birth rates. The significance of such processes can be assessed from the spatio-temporal pattern of microhabitat use (SCHINDLER 1988): The size of microhabitat patches was mostly much smaller than the animals´ range of dispersal or migration. And the microhabitat quality changed frequently throughout the lifespan of an animal (due to temporal water cover, desiccation, stiffness...). Such a microhabitat use generally requires a direct response of each animal to the ecophysiological conditions as discussed above, whereas other impacts are less significant (SCHINDLER 1988).
Only Evernia prunastri was rather continuously colonised, especially by Collembola. Even if the trunk is sprayed artificially, the animals do not leave the thalli of Evernia prunastri, whereas they do leave the bark crevices (PRINZING & WIRTZ in press). Furthermore, in Evernia prunastri several potential competitors, predators (Entelecara penicillata) or entomophagous fungi (mainly Trichoderma viride and Beauveria bassiana) were frequent. All these properties of Evernia prunastri make competition and natural enemies more significant for population regulation (SCHINDLER 1988). And indeed, thalli of Evernia prunastri can be recognisably overgrazed (LAUNDON 1971, PRINZING & WIRTZ in press), which automatically creates competition. The effects on the lichen will be discussed in the next section.
Also, the age-class distribution in different microhabitat types was rarely consistent with explanations other than the mentioned tolerance to desiccation and the ability to break up stiff cryptogams:
a) Reproduction (which does occur on tree bark in Cerobasis guestifalica and in entomobryid Collembola, JENTSCH 1940, BÜCHS 1988, MÜLLER-KRAENNER 1990) should make microhabitat use of oldest and youngest animals most similar: The latter hatch where the former stay for egg laying. Such a pattern was at most realised in Entomobrya nivalis' use of vertical crevices and, slightly, Orchesella cincta's use of horizontal crevices. The discrepancy in the other cases is well explicable: eggs should be deposited at sites which are permanently sheltered from waterfilms (JENTSCH 1940, MÜLLER-KRAENNER 1990) and also from entomophagous fungi. In contrast, juveniles should stay where desiccation is lowest (JENTSCH 1940, for Cerobasis guestifalica). Correspondingly, many of the eggs of Cerobasis guestifalica were found on the hydrophobic crusts of Lepraria incana and Pertusaria albescens, whereas hatchlings were primarily found in vertical crevices (Fig. 18, page 51).
b) Both, predators and the diameter of cavities effect animals size specifically and should thus make microhabitat use of similar-aged animals more similar. This might have been realised in Entomobrya nivalis' use of Lepraria incana, but all other significant patterns were contradictory.
4.3.3. Conclusions: adaptive value and prerequisites of microhabitat use
The observed colonisation of microhabitats can indeed be considered as an active and very important strategy to exploit food and climatic microshelters in this apparently unsheltered and unproductive macrohabitat exposed tree trunk because: (1) Most species differentiated accurately between even very similar microhabitat types. (2) The use of microhabitats was largely independent of the cryptogam's patch size or frequency - opposite to "classical" (phanerogam-) herbivores, which switch opportunistically (CRAWLEY 1983). Probably, the corticolous grazers could not afford switching because the respective preferred cryptogam species was also essential as shelter from drought, heat or drowning. Species which required less microshelter because they colonised the exposed tree trunk largely under macroclimatic shelter seemed to invest less in a differentiation between microhabitat types (Xenillus discrepans, Reuterella helvimaculata). (3) The colonisation of each microhabitat depended on distinct climates. This could mostly even be confirmed by comparison between independent scales, between species of similar physiological needs and between univariate and multivariate analyses. (4) The microhabitat use was explicable by the needs of the respective species. The pattern of microhabitat use of e. g. Entomobrya nivalis would not have been explicable when found e. g. in Carabodes labyrinthicus. (5) Other possible causes for such differentiated colonisation of microhabitats were largely insignificant. All this permitted the animals to improve their microhabitat conditions actively at any position and thus to use climatic gradients wherever it was beneficial (as discussed in the previous sections).
A species’ strategy of microhabitat use was probably not phylogenetically predetermined: species of very different taxa were sometimes quite similar in their average microhabitat use (Tab. 5). Instead, the species' use of microhabitats was probably adapted to the specific conditions on trunks.
Preadaptations for such a strategy to cope with extreme trunk environments might be minute size and eurytopy: The small microhabitat patches offer only little food and provide favourable climate only within the thin atmospheric boundary layer. Both can only be used by minute organisms. Furthermore, eurytopic species should be most capable of such a flexible use of microhabitats. This included habitat changes on very different scales: between different zones within a thallus of Evernia prunastri (PRINZING & WIRTZ in press), different microhabitat types, different microrelief zones or trunk faces and between the exposed trunk and neighbouring habitats such as trunk base, litter and crown (see discussion on climate use). The importance of minute size and eurytopy for the colonisation of trunks is
confirmed (1) by the dominance of eurytopic microarthropods as grazers (NICOLAI 1985, BÜCHS 1988, PRINZING & WIRTZ in press), (2) by the restriction of the largest species (Porcellio scaber) to a very short season of trunk colonisation, (during the present investigation to two warm and humid months in autumn), (3) by the comparison to Collembola in another extreme habitat: in cushions on alpine boulders. There, the fastest and most accurate migrators are most successful, too (BAUER & CHRISTIAN 1993).
4.4. Morphogenesis of the heavily grazed and wind-exposed lichen Evernia prunastri
The colonisation of the lichen Evernia prunastri was a substantial element of most grazers’ adaptive microhabitat use and thus a prerequisite for the exploitation of microclimatic benefits on trunks. Did this have a feedback effect on the grazed lichens? Grazing was considered especially capable of influencing the morphogenesis of the growth form, which by itself might influence the risk of windfall and desiccation for a thallus on such a wind-exposed substrate. Many of these growth forms were largely built up of branches with deviations from the even, flat, isotomous-dichotomous growth pattern (e. g. thalli in Fig. 22, 25 and PRINZING 1992). The causes of this variability in branch growth shall be discussed first. Afterwards, the processes shall be explained which imprinted the actual shape into which the growth form of a whole thallus developed.
4.4.1. Growth of branches
Observation of single branches revealed a close correlation between their cross-sections, their growth layers and branching patterns: A strong deviation from the horizontal inclination and orientation was induced by corners along edges of branches, an anisotomous-polytomous branching pattern by corner- or crater-like injuries at the branch tips (Fig. 27: (1), page 103). Such corners along edges or tips of branches were themselves induced by injuries. This confirms the observation of anisotomous-polytomous ramificaion patterns of branches as an effect of injuries at branch tips by STONE & McCUNE (1990).
Only grazing, natural or simulated, was observed to cause these injuries and irregularities of branch growth (Fig. 27: (2)). Theoretically, further causes might have been wind or any particles driven by wind, e. g. sand or ice-crystals, (Häckel 1993). But grazing seemed to be the prevailing impact, because of (1) the coincidence in the distributions of irregular growth and of grazing but not of wind (Tab. 9, page 101, comparisons between different thalli from PRINZING 1992) and (2) the structural similarity between feeding traces and corners along edges/destroyed tips of branches.
On the other hand, where the growth of branches was not disturbed by grazing, they largely developed symmetrical cross-sections, rounded edges, in a constant growth layer and with isotomous-dichotomous ramifications (Fig. 27 -(3), Table 9).
A third occasional type of asymmetries in branch cross-sections are strong infoldings of basal branches leading to extreme bending (PRINZING 1992). These basal branches were hidden from the photographic observation in the present study. Such asymmetries are often observed to be correlated with mining of juvenile Carabodes (PRINZING 1992). These animals then enlarge the tubes in the medulla, thereby increase the risk of a rupture toward the upper side and enhance the morphogenetic effect of such cross-sections on further branch-growth. Similar cases of mining by oribatids in the medulla of lichen thalli including ruptures to the upper side are described by Travé (1963) and - for fruticose thalli similar to Evernia prunastri - by Bachmann (1929). The latter author also shows how such tubes lead to strong infoldings and deep groves in the branch cross-section. Moreover, branches of fruticose lichens also become hollow and wider when mined (Zopf 1907). It therefore becomes probable that also in Evernia prunastri the juvenile Carabodes can not only enhance the effect of tube formation and infoldings in branches, but even induce them.
Table 9: Correlations of wind exposure, grazing and their combination with the predominant growth of branches and on the shape of complete thallus growth forms (summarised); "regular" growth of branches = mainly isotomous-dichotomous, even and flat .
wind exposure: sheltered thalli exposed thalli
grazing- (see material & methods)
intensity:
very low:
- on outer branches - growth of branches: mostly
of wind-exposed regular(see Tab. 7, Fig. 22)
thallus face
- on thalli with growth of branches: regular ...branches: regular, partially
dense algae-cover leewardly bent
(from PRINZING 1992, growth of complete thalli: ...complete thalli: regular and
observed in all 65 such thalli ) regular and loose loose, partially leewardly combed
normal: ...branches: with numerous irregularities (see Tab. 7, Fig. 22)
(E. prunastri without or ...complete thalli: rather ...complete thalli: dense lay-
sparse algal cover) loose, mainly the wind-shel- ers, mainly on the wind-exposed
tered face face
very high: ...branches: with extremely numerous irregularities
on E. p. herinii ...complete thalli: very ...complete thalli: very dense-
(from PRINZING 1992, irregular, mainly loose- bushy, almost closed at the
observed in all 20 such thalli) bristly outside
4.4.2. Growth form of whole thalli
The growth form of complete thalli, especially the growth-density, strongly matched the wind exposure - but only as long as the branches are also grazed upon (PRINZING 1992, Table 9). Without grazing, wind exposure did not have any effects on the growth form besides a bending of branches when moist (PRINZING 1992). Without wind-exposure (on wind-sheltered faces of thalli or trunks or in bark valleys), all growth forms were rather loose and susceptible to air currents.
The described variation in growth form even on differently exposed faces within the same thallus would have hardly been conceivable if all the single branches had been growing evenly and isotomous-dichotomously. Instead, such differentiated growth forms were able to develop, if (1) branches, including the young ones, were variable in their growth (Fig. 27: (4)) and (2) among such branches those increased fastest which grew into a wind-sheltered direction (Fig. 27: (5)). Both of these conditions could be demonstrated.
Interestingly, the variability was mainly generated by the disturbing effect of grazing. The selective effect of wind could have been due to (i) convective desiccation of the poikilohydric thalli, (ii) minute cracks in the cortex or its superficial erosion as a result of microscopic abrasion, (iii) ruptures of the cortex when branches are bent by wind or (iv) to the stress-effects of shaking. All such mechanisms have already been demonstrated for phanerogams (Nobel 1981). Convective cooling by wind might have additionally reduced the metabolism while the thalli were still wet, whereas it could not have led to serious chill-damage since this occurs in lichens only at extremely low temperatures (Henssen & Jahns 1974).
In the long run, these Darwinian processes of variation and selection led to thallus growth forms that were adapted to wind (Fig. 27: (6)). They were much less endangered by windfall than thalli of non-wind-adapted growth forms showing growth patterns which were too regular and simple (Fig. 27: (7)). The aerodynamic effect of such wind-adapted, dense growth forms is demonstrated by a desiccation experiment (Prinzing & Wirtz in press): wet thalli are dried with a hair drier from a fixed position and distance in front of the thallus for one minute. The percentage of water loss indicates the wind accessibility and turbulence on the thallus surface. It perfectly correlates to a decreasing growth density of the thallial frontal faces (rs = - 0.96, n = 15).
The basic adaptive effect of thallus growth forms is apparently the same as in phanerogam-crowns (Nobel 1981): an aerodynamic shape with a mutual sheltering of branches. The basic processes of adaptive morphogenesis (simple growth patterns develop adaptive polymorphism due to environmental disturbances and selection) are analogous to the phanerogams’ root system (HallÉ, pers. commun.).
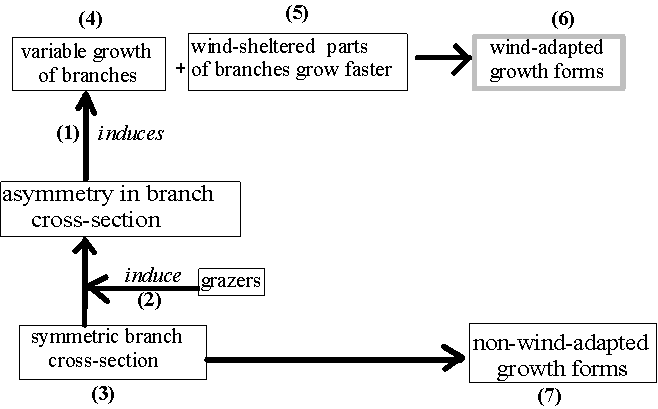
Figure 27: Interaction of processes determining the growth form of Evernia prunastri-thalli. Numbers are referred to in the discussion. Wind-adapted growth forms of whole thalli develop due to a faster growth in those parts of branches directed into wind-shelter at the respective microsite. The necessary supply with variation in growth directions is largely generated by grazing.
4.4.3. Fruticose (shrub-like) growth in other cryptogams
Evernia prunastri is not the only fruticose cryptogam on surfaces exposed to turbulent currents. How can the other species develop stable thalli? In a large number of taxa, thalli can generate a high variability in branch growth on their own, without additional disturbance by grazers. This can be due to cylindrical cross-sections of branches that permit ramifications into all orientations (e.g. the lichen-genera Usnea, Cladonia, Stereocaulon, mosses and some calcareous red algae). Fruticose cryptogam species with more flattened branches often have apical, spirally dividing cells (Phaeophyceae) or actually consist of bunches of cell-threads each dividing dichotomously but oriented into a separate direction (multiaxial Rhodophytina).
Many species of benthic fruticose algae lack both these features. Their growth forms are correspondingly even, often fan-like and very susceptible to water current. And in fact such thalli do not resist the water movement but adapt to it via their flexible, water-saturated bodies. The risk of detachment is reduced by a cartilaginous, leather-like consistency. Moreover, populations of many marine benthic shrub-like algae can easily compensate for occasional detachment because they are able to grow much faster than lichens in terrestrial, desiccating environments.
In all these examples grazing is not necessary for a morphogenesis of current-tolerant thalli. But there are still many fruticose, terrestrial cryptogams with flat branches, lacking spiral growth-patterns. It would be very interesting to find out
their strategies to cope with wind exposure and to test the importance of grazing. The existing qualitative descriptions of grazer-induced variation in such fruticose lichens (BACHMANN 1929, ZOPF 1907) suggest that the mechanisms might be similar such as in Evernia prunastri.
Compared with the indirect beneficial effects of grazing on Evernia prunastri, the detrimental direct effect might be of subordinate importance for those thalli that grow on strongly wind-exposed sites. Exactly here the grazers also prefer Evernia prunastri most! A completely detrimental effect of grazing has until now only been found under wind shelter on trunks in forests (extreme overgrazing reported by LAUNDON 1971). Mostly, however, the thalli are protected because grazing is always restricted to certain specific regions within a thallus shrub depending on the surrounding climate (Prinzing & Wirtz in press).
4.5. Resumé
The biocoenosis in this habitat, exposed tree trunks, is a simple, easily observable system. Such simple systems are a main opportunity to understand the complex habitat-animal relationships (for microarthropds: USHER et. al. 1982). The present study enabled me to understand the following spectrum of such relationships by applying a "microgeographic" perspective and by interpreting the results in the light of the physiological limits of the involved species:
(1) the effects of the habitat-topography (e. g. the bark microrelief) and its
vegetation cover and surface structure on the arthropods' living conditions;
(2) the arthropods' ability to use all these effects by a variety of adaptive
spatio-temporal distributions and their correlates in life history and physiology;
(3) the arthropods' feedback effect on a part of the vegetation (namely on the
adaptability of thallus-differentiation in space) depending again on the habitat
topography