3. Results
3.1. Analysis of microclimatic living conditions on exposed tree trunks
The absolute microclimate at a point of a trunk and its relation to the macroclimate depend on climatic patterns surrounding this point. From the analysis of this interaction one can infer (1) the underlaying climatic processes and (2) the patterns to which the animals’ distribution should correspond in order to maximize the microclimatic heat or humidity supply (absolutely or in relation to the macroclimate).
3.1.1. Absolute microclimatic conditions
3.1.1.1. Multiple regression analysis (Tab. 1):
Multiple regression analysis extracted the effect of each variable, given that the other variables remain constant.
Temperatures at 2 mm distance from the bark ("trunk temperatures") were absolutely high, mainly: (1) due to upheating of the trunk compared to the macroclimate (at 2 m distance from the trunk), (2) during warm macroclimate, (3) on warmer trunk faces and those of decreased water vapour pressure (variables mentioned in sequence of decreasing importance, Tab. 1: temperature A, B). Thus, the climatic effect of the trunk (or its zones) accounted for three out of four dominating variables. The differences between trunk climate and macroclimate are further analysed in 3.1.2., the climatic trunk zonations in 3.1.3.
The two variables "trunk/macroclimate difference of temperature" and "temperature of the macroclimate" were not included simultaneously in one analysis. Otherwise, they would have been perfectly but circularly correlated to trunk temperature and would have obscured the effect of other variables.
Inclusion of "trunk/macroclimate difference of temperature" (Tab. 1: temperature A) resulted in two kinds of misleading correlations of "trunk temperature" with: (a) a decreasing macroclimatic water vapour pressure (not significant) - probably as an effect of simultaneously often increased macroclimatic temperatures; (b) high wind speed - probably an effect of the position in the global west wind zone where the fastest winds come from the west and provide temperate air masses carried from the Atlantic ocean, in contrast to the slower east-winds that carry air masses from the cold north Asian winter; (c) sun shelter in the microrelief (p = 0.045, only) - but
Table 1: Multiple regression analyses on temperatures and saturation deficits, measured at a height of approx. 2 mm above the trunk. Not all variables could be tested at once because of circularity among variables (see text).
r.coeff.= standardised partial regression coefficient; toler. = tolerance, high to medium tolerances indicate a low degree of multicollinearity between the independent variables and thus high reliability of the coefficients; probab. = error term: (*), *, **, *** = p < 0.065, 0.05, 0.01, 0.001. Bold, underlined numbers indicate (nearly) significant interactions. The analysis as a whole is characterized by the number of investigated zones of exposure (N), by the explained variance "squared multiple R" and by an analysis of variance ANOVA. Wind speed in Beaufort; daytime as either 0 = night or 1 = day; weather as 1:<70 % cloud cover / 2: >70 % cover / 3: rain or soon after (water film still persisting on trunk).
|
variable |
influence on .... |
|||||||||||
|
...temperature |
...saturation deficit |
|||||||||||
|
A |
B |
A |
B |
|||||||||
|
r.coeff. |
toler. |
probab. |
r.coeff. |
toler. |
probab. |
r.coeff. |
toler. |
probab. |
r.coeff. |
toler. |
probab. |
|
|
macroclimate in 2 m distance: |
||||||||||||
|
® weather |
- .018 |
.468 |
- .103 |
.492 |
*** |
- .033 |
.472 |
- .268 |
.623 |
*** |
||
|
® daytime |
- .013 |
.774 |
.034 |
.780 |
(*) |
- .053 |
.775 |
* |
- .058 |
.774 |
* |
|
|
® wind speed |
.053 |
.911 |
*** |
- .012 |
.882 |
.001 |
.890 |
- .031 |
.881 |
|||
|
® temperature |
omitted |
---- |
---- |
.397 |
.143 |
*** |
.127 |
.607 |
*** |
1.000 |
.115 |
*** |
|
® water vapour pressure |
- .044 |
.396 |
(*) |
- .020 |
.393 |
- .539 |
.401 |
*** |
omitted |
---- |
---- |
|
|
difference trunk/macroclimate: |
||||||||||||
|
® temperature |
.325 |
.400 |
*** |
omitted |
---- |
---- |
.475 |
.406 |
*** |
.737 |
.318 |
*** |
|
® water vapour pressure |
.762 |
.559 |
*** |
.444 |
.134 |
*** |
omitted |
---- |
---- |
- .707 |
.107 |
*** |
|
difference between opposite trunk faces: |
||||||||||||
|
® temperature |
.135 |
.312 |
*** |
.350 |
.463 |
*** |
.268 |
.311 |
*** |
.111 |
.290 |
** |
|
® water vapour pressure |
- .134 |
.639 |
*** |
- .106 |
.594 |
*** |
- .239 |
.664 |
*** |
- .107 |
.555 |
*** |
|
trunk face exposed to: |
||||||||||||
|
® wind |
.011 |
.847 |
- .006 |
.846 |
.017 |
0.847 |
.000 |
.846 |
||||
|
® sun |
- .004 |
.641 |
.023 |
.643 |
- .065 |
0.641 |
** |
- .071 |
.641 |
* |
||
|
® precipitation |
- .005 |
.788 |
- .002 |
.788 |
.011 |
0.788 |
- .007 |
.788 |
||||
|
difference between opposite microrelief zones:® temperature |
.003 |
.842 |
.058 |
.868 |
*** |
.012 |
.842 |
- .004 |
.845 |
|||
|
microrelief zone exposed to: |
||||||||||||
|
® wind |
- .001 |
.761 |
- .003 |
.761 |
.002 |
0.762 |
- .077 |
.796 |
** |
|||
|
® sun |
- .032 |
.821 |
* |
.011 |
.813 |
.002 |
0.817 |
.039 |
.813 |
|||
|
® precipitation |
.002 |
.100 |
.012 |
.838 |
.007 |
0.837 |
.021 |
.839 |
||||
|
N |
384 |
384 |
384 |
384 |
|
squared multiple R |
0.925 |
0.905 |
0.867 |
0.804 |
|
ANOVA: F-ratio / probab. |
303.0 / *** |
234.7/ *** |
159.4 / *** |
100.4 / *** |
only when keeping the "trunk/macroclimate difference of temperature" (a much more significant variable) constant.
Inclusion of the "temperature of the macroclimate" resulted in a lower explained variance ("squared multiple R" in Tab. 1: temperature B). Therefore, the positive correlations of "trunk temperature" with daytime, sunny weather and warming of the microrelief zone were probably acting mainly indirectly via the "trunk/ macroclimate difference of temperature", which had been included in the previous analysis.
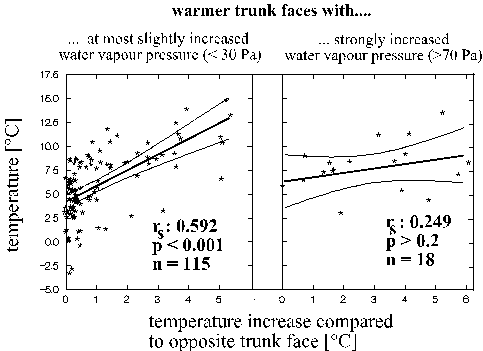
In both analyses, "trunk temperature" was correlated to increased water vapour pressures at the bark compared to the macroclimate. However, this must have been an effect of heat (not its cause): an increased evapotranspiration of water vapour from the warm substrate. Such evapotranspiration could by itself only cause a reduction of temperatures (examples for soils in JUUSELA 1945, JACOBSEN et. al. 1953). Evapotranspirative cooling was indeed only significant on the trunk faces of relatively increased water vapour pressures compared to the opposite faces. The univariate analysis of this pattern (Fig. 3) shows that the evapotranspirative cooling could usually compensate the effect of upheating of the same trunk face only on trunk faces with highly increased water vapour pressures.

Figure 3: The effect of temperature increase on a trunk face (compared to the opposite face, x-axis) on absolute heat (y-axis). This effect only became compensated when the water vapour pressure increased strongly between these trunk faces, too (right graph, in contrast: left graph). In order to ensure similar macroclimatic backgrounds, only measurements during 0 - 10°C of the macroclimate in 2 m distance were considered. Trunk faces of more than 6.5°C temperature increase were not considered because they never coincided with < 30 Pa water vapour pressure increase.
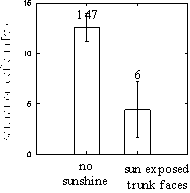
Saturation deficits at 2 mm from the trunk were lowest mainly: (1) when the macroclimate was rich in water vapour and, additionally, cool, (2) when the trunk was cool and enriched in water vapour compared to the macroclimate (further analysed in 3.1.2.), (3) on trunk faces of lower temperatures, higher water vapour pressures (further analysed in 3.1.3.) and, additionally, sun exposure and (4) during the day (Tab. 1: saturation deficit A, B). The negligible effect of exposures to precipitation (i. e. waterfilms) corresponded to the generally saturated atmosphere on any side of a trunk during rain that is strong enough to cause water films on certain trunk faces. The significant reduction of saturation deficits by mere sun exposure (when its temperature effects are kept constant) was amazing. Nevertheless, this effect could be confirmed univariately when considering only trunks which are not warmer than the macroclimate (Fig. 4).
In order to prevent circularity among independent and dependent variables it was not possible to include both the water vapour pressure of the macroclimate and its relative change towards the trunk into the same multiple regression analysis. Inclusion of the latter variable yielded a lower sqared multiple R value (Tab. 1: saturation deficit B). Thus, the additional positive effects of rainy weather and of the microrelief's exposure to wind probably only indirectly reflected the more direct effect of the macroclimate's water vapour pressure.
![]()
Figure 4: Effect of sun exposure of a trunk face on saturation deficits (means + 1 SEM) - given that the trunk was not upheated compared to the macroclimate. The decrease in saturation deficit was not significant (due to the low number of measurements of sun exposed but not upheated trunk zones), but it confirmed the significant results in the multiple regression analysis (Tab. 1: saturation deficit A and B). Only measurements under a comparable range of saturation deficits were considered (0-66 Pa (= 0.5 Torr)).
3.1.1.2. Univariate analysis of categorial factors:
Monthly means of temperatures showed a rather continuous seasonal development, except for a sudden decrease just before the onset of spring (Fig. 5). Such a decrease occurs most years in Germany. Changes in mean saturation deficits were not parallel to the temperatures except that the two warmest months were also the driest (Fig. 5). Fluctuations of temperature but not of saturation deficits seemed to be buffered in winter. Conditions in August and September are generally also representative for May and July (which were not investigated): average temperature and precipitation are only 3 and 7 % lower in May to July (HÖRMANN et. al. 1992). At night, temperature and saturation deficits decreased significantly (Fig. 6). The latter was primarily an indirect effect of the thermal conditions during night (see above multiple regression analysis).
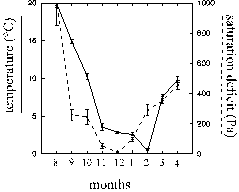
Figure 5: Monthly changes of trunk climate (means of all measurements at 2 mm above the bark, + 1 SEM)
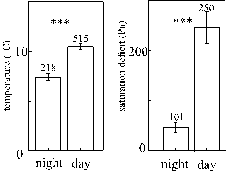
Figure 6: Diurnal changes of trunk climate (means of all measurements at 2 mm above the trunk from August to December, + 1 SEM). Numbers of measurements are indicated on top of each bar.
3.1.2. Climatic differences between the trunk and the macroclimate
3.1.2.1. Multiple regression analysis:
Multiple regression analysis extracted the effect of each variable, given that the other variables remain constant.
Temperatures at 2 mm from the trunk were higher than those of the macroclimate at 2 m distance ("upheating of the trunk") (1) during low macroclimatic temperatures, (2) on the trunk faces of higher temperature and, additionally, of lower water vapour pressure than the opposite trunk face (further analysed in 3.1.3.), (3) on warmer microrelief zones, (4) during sunny weather and (not significant) during the day (Tab. 2: temperature). The positive correlation with "trunk/macroclimate differences of water vapour pressure", however, must be regarded as an effect of the trunk's upheating (an accelerated evapotranspiration of water from the upheated substrate) and not vice versa (see above multiple regression analysis of "trunk temperatures").
Table 2: Multiple regression analyses on differences of temperature and water vapour
pressure between approx. 2 mm above trunk surface and the macroclimate in approx. 2 m distance.
For further explanations see Tab. 1.
|
variable |
influence on trunk/macroclimate difference of .... |
|||||||
|
temperature |
water vapour pressure |
|||||||
|
r.coeff. |
toler. |
probab. |
r.coeff. |
toler. |
probab. |
|||
|
macroclimate in 2 m distance: |
||||||||
|
® weather |
- .188 |
.492 |
*** |
.049 |
.472 |
* |
||
|
® daytime |
.062 |
.780 |
(*) |
.019 |
.775 |
|||
|
® wind speed |
- .023 |
.882 |
- .034 |
.890 |
(*) |
|||
|
® temperature |
- .760 |
.143 |
*** |
.865 |
.607 |
*** |
||
|
® water vapour pressure |
- .036 |
.393 |
- .075 |
.401 |
** |
|||
|
difference trunk/macroclimate: |
||||||||
|
® temperature |
dependent variable |
.268 |
.406 |
*** |
||||
|
® water vapour pressure |
.812 |
.134 |
*** |
dependent variable |
||||
|
difference between opposite trunk faces: |
||||||||
|
® temperature |
.640 |
.463 |
*** |
- .158 |
.311 |
*** |
||
|
® water vapour pressure |
- .195 |
.594 |
*** |
.177 |
.664 |
*** |
||
|
trunk face exposed to: |
||||||||
|
® wind |
- .010 |
.846 |
- .011 |
.847 |
||||
|
® sun |
.043 |
.643 |
.007 |
.641 |
||||
|
® precipitation |
- .004 |
.788 |
.008 |
.788 |
||||
|
difference between opposite microrelief zones:® temperature |
.106 |
.868 |
*** |
.007 |
.842 |
|||
|
microrelief zone exposed to: |
||||||||
|
® wind |
- .005 |
.761 |
.014 |
.762 |
||||
|
® sun |
.021 |
.813 |
.026 |
.817 |
||||
|
® precipitation |
.022 |
.838 |
.008 |
.837 |
||||
|
N |
384 |
384 |
|
squared multiple R |
0.683 |
0.683 |
|
ANOVA: F-ratio / probab. |
52.8 / *** |
52.8 / *** |
Water vapour pressures at 2 mm from the trunk were higher than in the macroclimate (1) when the macroclimate was warm and, additionally, contained little water vapour, (2) when the trunk was upheated compared to the macroclimate, (3) on trunk faces of higher water vapour pressure and lower temperatures compared
to the opposite face (further analysed in 3.1.3.). Small effects were due to rainy weather or low wind speed (almost significant) (Tab 2: water vapour pressure).
All these mentioned conditions favoured an efflux of water vapour from the trunk towards the lower concentrations. Such fluxes could not be inferred from mere saturation deficits at the trunk since the water content and temperature inside the bark are not known (STOUTJESDIJK & BARKMAN 1992) and could practically not be investigated simultaneously to the conditions in the air.
The variables temperature and water vapour pressure of the macroclimate covered an especially large spectrum of values. Nevertheless, their combined effect (the macroclimatic saturation deficit) on the differences of water vapour pressure between trunk and macroclimate was +linear (Fig 7 a). In contrast, the effect of wind on this difference changed: water vapour pressure increased towards the trunk only during a medium increase in wind speeds, but started to decrease during strong wind (Fig. 7 b). This general effect of different wind speeds did not change substantially during different weather conditions (Fig. 7 b) or different saturation deficits of the macroclimate.
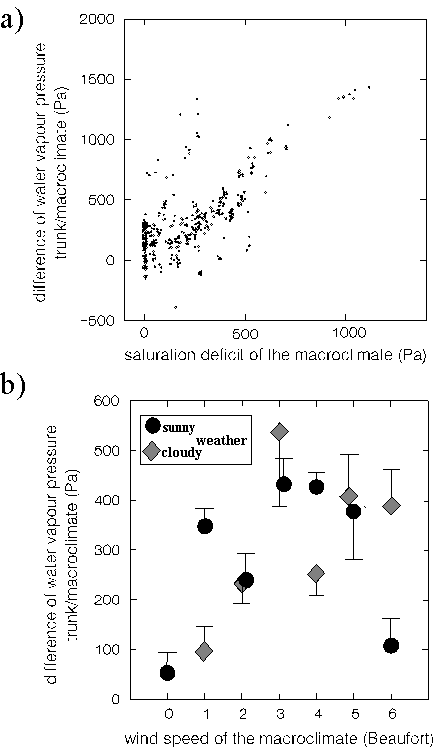
Figure 7 (legend on next page)
3.1.2.2. Univariate analysis of categorial factors
Absolute values of trunk/macroclimate differences of heat and of water vapour pressure both decreased and subsequently increased during winter, but they were otherwise rather different in their seasonality (Fig. 8 a).
Comparisons between tree species can be useful for discussion of the physical mechanisms by which the substrate might modify the microclimate (the animals' climatic living conditions on a tree species cannot be directly inferred, because the humidities in the bark crevices were not measurable). Among the trees with a normal epiphyte cover (< 70 % algae) and a normal exposure (see 2.1., page 8), oaks and ashes were thermally most different from the macroclimate, limes and also beeches least different (Fig. 8 b). Trees with strong algal cover were generally less different than their conspecifics. Also, the water vapour pressures at ash trunks were very different from the macroclimate, but in this respect oaks were least different. Water vapour pressures on very exposed trees were most different from the macroclimate and they were also more different on trees with a dominating algae cover compared to the conspecifics with less algae. Species-specific effects were less important.
Figure 8: Absolute values of differences of temperature and of water vapour pressure between trunk and macroclimate (measured at approx. 2 mm and 2 m distance from the trunk; means + 1 SEM). Comparison between different months (a) and types of trees (b). Tree types: 1, 2: oaks with normal or strong cover by algae (< or > 70 % cover), 3: extremely solitary oaks (definition see 2.1. page 8), 4, 5: beeches with normal or strong cover by algae, 6: ashes, 7: limes.
3.1.3. Climatic zonations at the trunk surface
Trunk face zonations of temperatures and water vapour pressures corresponded only slightly to the respective actual exposures of the trunk face (squared multiple R values of .36 and .02 in the respective multiple regression analyses), sun exposure being most relevant. Thus, zonations mainly depended on the trunk face's accumulation or loss of heat and water during the previous exposures. This was most extreme during the night (squared multiple R for temperature zonation: only .16).
The mean temperature differences between opposite trunk faces (Fig. 9) were not more than three times as high as those within the microrelief, although the former invoked much larger distances (> approx. 1 m) than the latter (approx. 2 - 8 cm). Differences were mostly below 3 and 1°C, respectively (Fig. 9). But sometimes they could reach up to 22 °C (8°C) between opposite trunk faces (microrelief zones).
Persistance of temperature differences under low to medium wind speeds was higher within the microrelief than between trunk faces (Fig. 9). Temperature difference between opposite trunk faces decreased more strongly during strong wind than the differences of water vapour pressures (Fig. 9). This was not found in the microrelief - possibly because, for technical reasons, only few humidity measurements had been conducted in bark valleys.
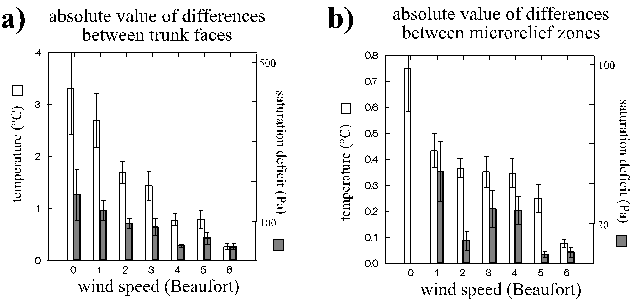
Figure 9: Absolute values of differences of temperature and water vapour pressure between opposite trunk faces (a) or microrelief zones such as bark ridges and bark valleys (b) of the trunk (means + 1 SEM) influenced by macroclimatic wind speeds.
Reduction of wind speed on the sheltered trunk face was rather similar on all considered species and types of trees (Fig. 10 a). On the microrelief scale, wind zonations corresponded nicely to the roughness of the trunk surfaces: highest on oaks and limes, moderate in ashes and lowest in beeches (those with strong algae cover were often younger and had a correspondingly smoother surface) (Fig. 10 b).
Despite the immense range of wind speeds encountered throughout the period of investigation (Fig. 10 c), the reduction of wind at the trunk remained rather constant, as indicated by the low standard errors in Fig. 10 a and b. And indeed, the measured absolute velocity of air even in sheltered, narrow valleys of the bark ranged from 0 up to 3 m/sec during a breese and a storm, respectively.
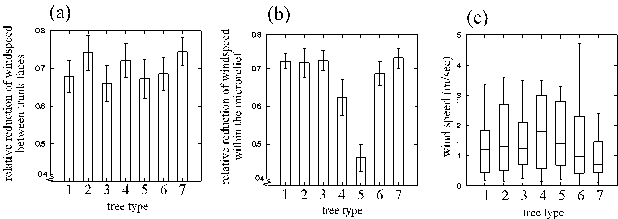
Figure 10: Relative reduction of wind speeds on different tree types between the exposed and sheltered trunk faces (a); and between exposed and sheltered microrelief zones (b); and range of wind speeds that the different tree types experienced at the completely unsheltered parts of their trunks (c). In (a) and (b) means + 1 SEM are given, in (c) the ranges, interquartiles and medians. Tree types: 1, 2: oaks with normal or strong cover by algae (< or > 70 % cover), 3: extremely solitary oaks (see 2.1. page 8), 4, 5: beeches with normal or strong cover by algae, 6: ashes, 7: limes.
3.1.4. An example of microclimatic patterns
Fig. 11 presents climate measurements at a trunk in March (including humidity measurements in bark valleys - the necessary techniques had been available then). The microclimate clearly differed from one position to the other - especially the saturation deficits (middle numbers in Fig. 11). The actual distribution of climates depended strongly on the previous exposure to sun: the east-facing positions 1, 2, and 3 had already been exposed to sun during the previous morning. This explained the higher temperatures on this trunk face compared to the positions 4, 5 and 6. The same, but less pronounced effect occurred between positions 9, 10 and 7, 8. The zones exposed to sun for the longest time (positions 2, 3) were considerably warmer than the macroclimate and the water vapour pressure was correspondingly higher, too. The corresponding evapotranspirative cooling did not seem to compensate this upheating. However, where the trunk was not warmer than the macroclimate the temperatures tended indeed to decrease with an increase in water vapour pressure (comparing positions 4 and 8 or 6/5 and 7).
All these interpretations were inferred from the above multiple regression analyses. They explained a pattern that, at first sight, seemed to correspond to wind exposure of the trunk face. But exposure to winds of 12°C and 998 Pa water vapour pressure could not have caused convective nor evapotranspirative heat loss from the wind-exposed parts of the trunk (positions 5, 6, 7) since they were even cooler and had a lower water vapour pressure. In addition, the wind exposure within the microrelief partly did not coincide with a cooling, either (comparing positions 5 to 4, 7 to 8 and 2 to 3). The exemplary presentation of complex climatic patterns in space and also throughout a day can easily end up in such pitfalls or with hardly interpretable complexes of interrelated factors. Both could be largely avoided by the employed procedures of sampling and statistical analysis.
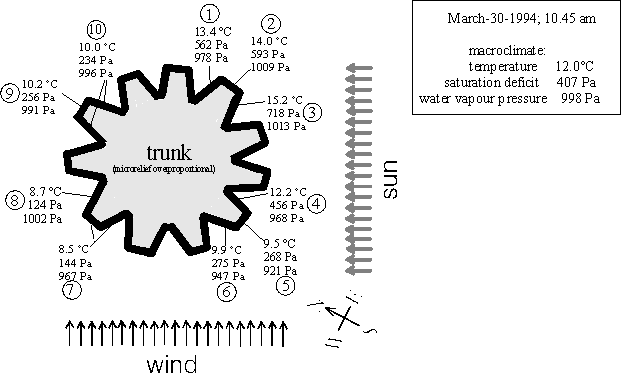
Figure 11: Microclimates at a tree trunk on a late morning of a sunny day in spring (scheme, the microrelief is strongly enlarged and simplified). The temperature, saturation deficit and water vapour pressure are given for each position and for the macroclimate (as upper, middle or lower number). Directions of wind and sun are indicated. Interpretation see text.
3.1.5. Balances of the direct and indirect effects of variables
Each variable could have several, partly indirect effects on the microclimatic heat (i. e. temperature or upheating) and humidity (i. e. low saturation deficits, or water vapour influx during a decrease of water vapour pressure towards the trunk). Path analysis balanced such influences (Tab. 3). For such a calculation I considered only variables that (1) had yielded a significant partial regression coefficient (see Tab. 1 and 2, and chapter 3.1.3. for temperature zonation), and (2) were also interpretable as direct causes of the respective dependent variable (see discussion in 3.1.1.1. and 3.1.2.1.). The path coefficients for wind speeds are only given in brackets due to the mentioned, non-linear effect on water vapour pressure differences (see Fig. 7 b).
Heat and humidity were not antagonistic in their variables with the highest path coefficient: Both upheating and influx of water vapour even corresponded to a cool macroclimate. The trunk/macroclimate difference in water vapour pressure was exclusively relevant to the saturation deficits. The trunk's temperature and also upheating interacted equally with the thermic trunk face zonation. Its effect on humidity was opposite but less dominant than on heat/upheating. And the water vapour influxes were hardly effected by the thermic trunk face zonation at all (Tab. 3).
Table 3: Balanced direct and indirect importance of different climatic variables for
relatively and absolutely high heat and humidity at the trunk microclimate, summarized
by path analysis from multiple regression analyses in Tab. 1 and 2 and section 3.1.3., discussed in 3.1.1.1. and 3.1.2.1.. For further explanations see Tab. 1.
|
variable |
balanced influence on ... (path coefficient / rank of variable) |
|||
|
heat |
low sat. deficit |
upheating of trunk |
decreased wat. vap. press. at trunk (->influx) |
|
|
macroclimate in 2 m distance: |
||||
|
® weather |
-.061 / 6 |
.001 / 11 |
-.188 / 5 |
.001 / 10 |
|
® daytime |
.020 / 8 |
.036 / 8 |
.062 / 7 |
-.017 / 7 |
|
® wind speed |
(-.024/ 9) |
(.034 / 5) |
||
|
® temperature |
.150 / 5 |
.702 / 2 |
-.760 / 1 |
-.661 / 1 |
|
® water vapour pressure |
.486 / 4 |
.075 / 4 |
||
|
difference trunk/macroclimate: |
||||
|
® temperature |
.325 / 2 |
-.286 / 6 |
dep. variable |
-.268 / 2 |
|
® water vapour |
.707 / 1 |
dependent variable |
||
|
difference between opposite trunk faces: |
||||
|
® temperature |
.343 / 1 |
-.562 / 3 |
.640 / 2 |
-.014 / 8 |
|
® water vapour pressure |
-.197 / 4 |
.420 / 5 |
-.195 / 4 |
-.125 / 3 |
|
trunk face exposed to: |
||||
|
® wind |
||||
|
® sun |
.206 / 3 |
-.274 / 7 |
.364 / 3 |
.008 / 9 |
|
® precipitation |
||||
|
difference between opposite microrelief zones:® temperature |
.034 / 7 |
.020 / 10 |
.106 / 6 |
-.028 / 6 |
|
microrelief zone exposed to: |
||||
|
® wind |
||||
|
® sun |
||||
|
® precipitation |
||||
3.2. Use of climatic gradients by corticolous arthropods
The microclimatic analysis in the previous chapter revealed which climatic patterns influence the animals’ microclimatic living conditions. The distribution of animal species will now be compared to these patterns. Moreover, seasonal weather conditions, daytimes and wind exposures will be considered as they correspond to the possible non-microclimatic benefits of trunk colonisation mentioned in the intro-duction. The climatic patterns consist of gradients, even though they have to be intersected into ordered categories in the following analysis. In contrast, the microhabitats which will be dealt with in part 3.3. are discrete, isolated structures. Different climates often correspond to different frequencies of certain microhabitat types (but not to a general absence of a type). Therefore, in the present analysis the animals´ abundances had to be related to a standard microhabitat composition (see 2.4.2., page 16). Such abundance values inevitably differ from those which are related to a trunk area selected randomly without respect to its microhabitat composition. The latter kind of values has already been extensively provided by other authors, e. g. TURNER 1983, BÜCHS 1988, NICOLAI 1985, BRAUN 1992, WUNDERLE 1992 a. Numbers of animals considered for comparison to a certain climate (Fig. 12) are given in the graphs. 3863 samples of microhabitats were investigated (18 cm2 each), see Fig. 17 for a differentiation of the types of microhabitats and their use by the different species.
3.2.1. Use of microclimate: heat, humidity, scales and zonations
Expectation: The species' distributions correspond (1) primarily to patterns that favour either heat, upheating, low saturation deficit or influx of water vapour (see Tab. 4 on page 36); (2) to several of these climates (i. e. to heat and water vapour influx) on different scales; (3) to the regular trunk face and microrelief zonations; or (4) to the regular climatic zonations perpendicular to the trunk surface, the "climatic profile": A shallow temperature gradient (defined as < 0.5°C difference between neighbouring microrelief zones) primarily effects the climate at the trunk surface; a steep gradient (> 0.5°C difference within the microrelief) also effects the climate several millimetres above and below the surface, i. e. in bark crevices (Fig. 13, page 39). Such thermic microrelief zonations were not only coupled to the climatic differences measured between the macroclimate and the bark surface (Tab. 2, 3; NICOLAI 1985), but also indicate the thermic profile between the interior and
the surface of the bark: Inner bark will mostly have an intermediate temperature inbetween those of the adjacent cooler and warmer zones of the bark surface. Correspondingly, there´s a decreasing / increasing thermic profile between the the inner bark and the cooler / warmer surface zones. Since a thermic microrelief zonation is realised and measurable in almost each trunk face of each tree its effects should also be well detectable, because (a) almost each animal has an opportunity to select between such zones at any time and (b) the pattern could be sampled very often, which permitted statistical analysis.
Observations:
C o l l e m b o l a: Orchesella cincta's distribution corresponded to climates on all scales (macroclimate up to microrelief climate, Fig. 12 a). High microclimatic humidities were favoured by distribution on two scales independently: the macroclimate’s water vapour pressure and the trunk/macroclimate temperature difference. Only the increasing densities during a warm macroclimate had an opposite but very small effect (small standardised partial regression coefficient in Tab. 4, page 36). Orchesella cincta also experienced (a) upheating due to the position on warm microrelief zones, (b) heat due to the warm macroclimate, and (c) water vapour influx due to the use of trunk areas cooler than the macroclimate (Tab. 4, Fig. 12 a, page 37). Microrelief distributions intensified rather than compensated the thermic effects of the respective trunk face (Fig. 12 a). During shallow temperature gradients in the microrelief (which mainly effect the climate at the trunk surface) a cooling corresponded to an increasing use of this trunk surface layer. During stronger gradients, however, no changes were observed at all (Fig 13, page 39).
In Entomobrya nivalis the distribution hardly corresponded directly to the macroclimate. The distribution, however, corresponded to conditions that favour an upheating of the trunk on two mutually independent microclimatic scales (Fig. 12 a, compared to Tab. 4, page 36): (1) the compensation of the trunk face's cooling within the microrelief and the use of trunk faces of lower water vapour pressure, and (2) the use of warmer zones of the microrelief. Nevertheless, the average densities were lower on upheated trunks (Fig. 12 a). This might be explained by a seasonal bias: in December the trunks were slightly cooled on average (in contrast to conditions during all other investigated months), but population densities were rather high (Fig. 12 b, page 38) - probably because of the still moderate temperatures (on average 3°C, Fig. 5, page 26) and because of the very humid air (average saturation deficits almost = 0 Pa, Fig. 5). In contrast, in February trunks were strongly upheated, but temperatures were lowest (on average ca. 0°C, Fig. 5) and the air was moderately dry (sat. def. on average ca. 350 Pa; Fig. 5). Then, the population densities were also very low. Such seasonal biases could have hardly been relevant for the other Collembola species, since they were rare during winter (Fig. 12 b, page 38).
Table 4: Patterns that influence the heat, upheating, humidity and influx of water vapour to which the animals are exposed to according to the multiple regression analyses (Tables 2 and 3 and discussion in 3.1.1.1. and 3.1.2.1.). Distributions of animals are compared to these patterns in Fig. 12 a (for weather conditions Fig. 12 c). Climatic patterns which had not yielded significant partial standardised regression coefficients or such with an absolute value < 0.075 are not considered for reasons of clarity.
|
variables |
relevance for trunk microclimate (standardised partial regression coefficients) with respect to: |
|||
|
(ordered according to scales) |
heat |
upheating |
low saturation deficit |
influx of water vapour |
|
macroclimate: |
||||
|
high temperature |
.397 |
-.760 |
-.127 |
-.865 |
|
high water vapour pressure |
--- |
--- |
.539 |
--- |
|
sunny weather (< 70 % cloud cover) |
--- |
.188 |
--- |
--- |
|
trunk /macroclimate differences: |
||||
|
trunk warmer |
.325 |
--- |
-.475 |
-.268 |
|
trunk with higher water vap. press. |
--- |
--- |
.707 |
--- |
|
trunk face zonation: |
||||
|
warm trunk face |
.135 |
.640 |
-.268 |
.158 |
|
trunk face with higher wat. vap. press. |
-.134 |
-.195 |
.239 |
-.177 |
|
microrelief zonation: |
||||
|
warm microrelief zone |
--- |
.106 |
--- |
--- |
Figure 12 (next two pages): Mean densities (+ 1 SEM) of the most frequent bark colonisers (rows) under different climates (columns). a) thermic and hygric conditions realised on different scales, (next page:) b) seasons, c) weather conditions and daytimes, d) exposures to wind dispersal. Every second row is shaded for reasons of clarity.
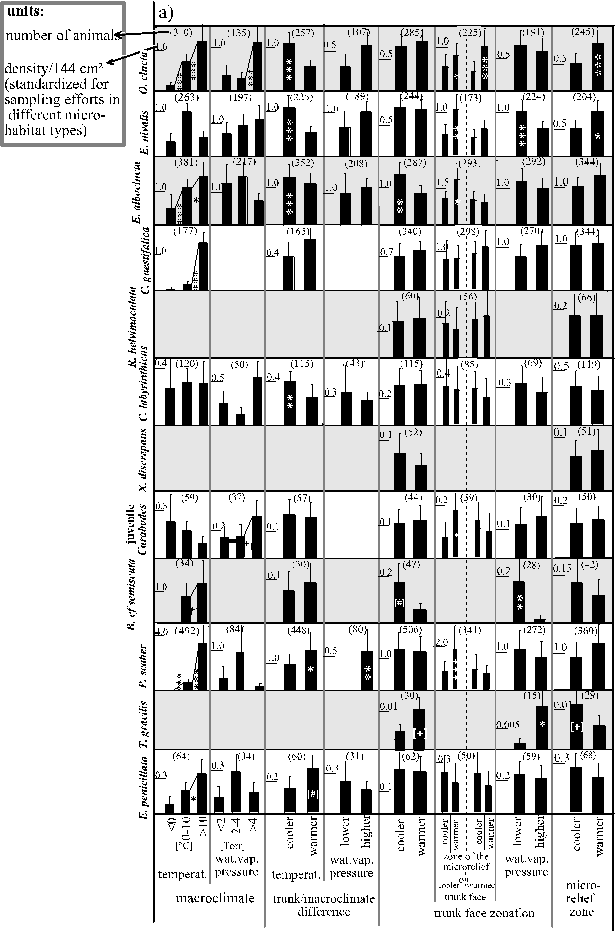
climates
Figure 12 a (legend on previous page)
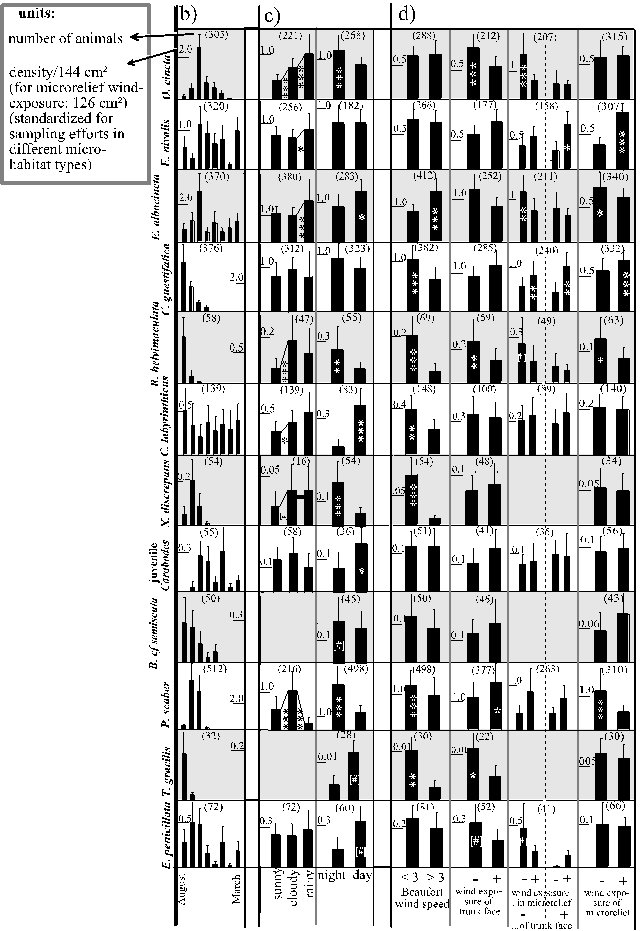
climates
Figure 12 b-d) (legend on page 36)
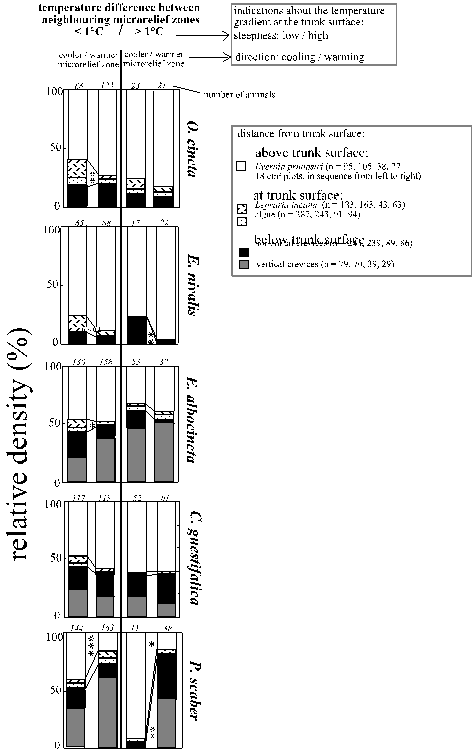
Figure 13: Distribution of the five most common arthropod species within the climatic profile perpendicular to the trunk surface. Relative density = (average density on a microhabitat type) : (sum of average densities on all microhabitat types). Proportional increases of a species' frequencies in Evernia prunastri, on bark surface microhabitats or in crevices are indicated as *, **, *** = p < 0.05, 0.01, 0.001 (Chi² test of goodness where applicable, otherwise Kolmogorov-Smirnov-test).
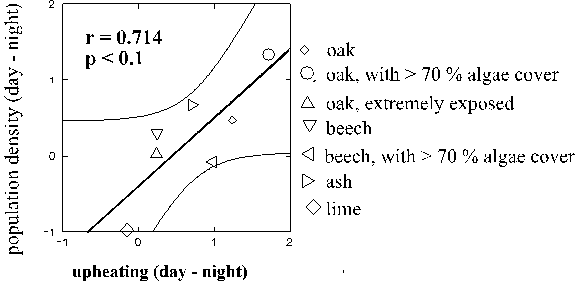
Figure 14: Diurnal abundance dynamic of Entomobrya nivalis in relation to upheating on different tree types (definition of "extremely exposed oaks" see 2.1., page 8). Difference between average diurnal and average nocturnal trunk / macroclimate temperature differences on x-axis (°C). Difference between average diurnal and average nocturnal densities/144 cm² on y-axis (calculated for a standard microhabitat composition, see Fig. 12). Linear smoothing, 95 % confidence limits, Spearman rank correlation and p-value are given.
This seasonal bias in Entomobrya nivalis was avoided in more differentiated comparisons: (a) the monthly sequence of changes in the trunks' upheating corresponded to the sequence of density changes in Entomobrya nivalis from September (when upheating measurements started) until January: upheating and densities increased from September to October, then decreased until December and increased to January. (b) On tree types of strong diurnal upheating Entomobrya nivalis' densities also increased diurnally (Fig. 14, this page, not fully significant due to the small sample size of only seven tree types). Both types of evidence were only found in Entomobrya nivalis. Entomobrya nivalis also experienced an influx of water vapour due to the mentioned use of trunk faces and due to the seasonal use of trunk areas cooler than the macroclimate. The compensation of cooling of trunk faces within the microrelief improved the heat supply (Fig. 12 a, page 37, compared to Tab. 4).
During shallow temperature gradients in the microrelief (which mainly effect the climate at the surface of the trunk) a cooling corresponded to an increasing use of this trunk surface layer. The crevices below were only used increasingly during a stronger cooling which effects the climate in a broader zone next to the trunk surface (Fig. 13, page 39).
Entomobrya albocincta's distribution corresponded to climates on all scales and almost uniformly to factors that independently favour a cooling of the trunk compared to the macroclimate: warm macroclimates, rainy weather and cooler trunk faces (Fig. 12 a, page 37, compared to Tab. 4). However, the increased densities on cooled trunk faces were compensated by an increased use of warmer microrelief zones (Fig. 12 a). In addition, Entomobrya albocincta experienced (i) heat due to the warm macroclimate; (ii) humidity due to the use of cooler trunk faces and of trunk areas cooler than the macroclimate (Fig. 12 a compared to Tab. 4); and (iii) influx of water vapour due to the latter distribution. During shallow temperature gradients in the microrelief (which mostly effect the climates at the surface of the trunk) a cooling corresponded to an increasing use of this surface layer. During stronger gradients, however, no changes were observed at all (Fig 13, page 39).
P s o c o p t e r a : Cerobasis guestifalica's distributions hardly followed the thermic regime (Fig. 12 a, page 37). High temperatures, cooled trunks and also an efflux of water vapour were favoured by the use of warm macroclimates only. The experienced saturation deficits were not modified by the use of the trunk faces and probably also not by the trunk/macroclimate gradients; macroclimatic effects on humidities could not be assessed (Fig. 12 a, compared to Tab. 4). The distance from the trunk surface did not depend on direction and steepness of the surrounding temperature gradients (Fig. 13, page 39). Reuterella helvimaculata only occurred during the warmest seasons investigated. But thermic zonations of the microclimate, which reflect the trunk's upheating, were irrelevant (Fig. 12 a, page 37 compared to Tab. 4). Sunny weather and daytimes were even avoided (Fig. 12 c). Further comparisons were not feasible due to the small number of sampled animals.
A c a r i : Microclimates of adult Carabodes labyrinthicus were only modified by rather large-scale distributional patterns: the decrease during sunny weather and on trunk zones cooler than the macroclimate. These patterns favour an exposure to cool, moist microclimates and to an influx of water vapour (Fig. 12 a, page 37 compared to Tab. 4). Xenillus discrepans did not correspond to thermic zonations (Fig. 12 a). Other microclimatic patterns could not be tested. Juvenile Carabodes2) were exposed (1) to upheating and drought due to the compensation of the trunk face's cooling within the microrelief and, simultaneously, (2) to moisture due to the used macroclimatic water vapour pressure (Fig. 12 a, compared to Tab. 4; Chi² = 2.5 (df = 1) considered due to the small number of animals sampled). Bdella cf semiscuta's use of cooler trunk faces and, independently, of warm macroclimates exposed the animals to a cooled trunk. The use of trunk faces of lower water vapour pressure had an opposite but much less significant effect. The use of warm macroclimates also favoured an efflux of water vapour (Fig. 12 a, compared to Tab. 4).
Distributions of the i s o p o d Porcellio scaber interacted with climates on all scales. Microclimatic heat was especially favoured by the distribution on three mutually independent scales: (1) warm macroclimate, (2) trunk zones of even higher temperatures than the macroclimate, and (3) a compensation of the trunk face's cooling within the microrelief (Fig. 12 a, page 37, compared to Tab. 4). Simultaneously, the animals were exposed to an efflux of water vapour due to distributions (1) and (2). During steep temperature gradients in the microrelief, which mostly effect the climate of a broad layer next to the trunk surface, an upheating corresponded to an increasing use of the crevices below the trunk surface. The use of the trunk surface increased slightly when exposed to an only shallow increase of temperatures. Evernia prunastri was used otherwise (Fig. 13, page 39).
Temnostethus gracilis ( H e t e r o p t e r a ) corresponded to climates on all scales (Fig. 12 a, page 37; considering also Chi² values of 2.6 and 2.5 (1 df) due to few sampled animals). The distribution did not effect the thermic or hygric environment unequivocally on more than one scale. Temnostethus gracilis was exposed to upheating due to the used warm trunk faces and simultaneously to heat and to water vapour effluxes due to the restriction to the macroclimatically warmest months (Fig. 12 a and b, compared to Tab. 4). Too few animals had been sampled during measurements of differences between trunk and macroclimate. Entelecara penicillata ( A r a n e a e ) corresponded to warm macroclimates and to an upheating of the trunk zone compared to the macroclimate. Both independently favoured an exposure of the animals to heat and drought and to an efflux of water vapour (Fig. 12 a, page 37, compared to Tab. 4).
3.2.2. Use of seasonal climates: opportunities for migration between soil and crown
Expectation: Trunk colonisation is coupled to the living conditions in the crown - thus to the tree's vegetation period. A dispersive, probably subadult stage dominates (as found in migratory macroarthropods by ALBERT 1976 and ADIS 1984 and in migratory microarthropods by CHRITENSEN (1980) and, in tendency, ALLMEN & ZETTEL (1983)).
Observations: Cerobasis guestifalica, Reuterella helvimaculata, Xenillus discrepans, Porcellio scaber and Temnostethus gracilis occured on the trunks largely during the vegetaion period of the tree (lasting until October). However, the exact population densities changed strongly throughout this period (Fig 12 b, page 38). The end of the vegetation period corresponded to extraordinary increases in densities of Orchesella cincta and Entomobrya albocincta. But even in these species, all age-classes were represented at that time, and adults were the most frequent (Fig. 15). Also in the other analysed species (Cerobasis guestifalica, Entomobrya nivalis) all age-classes were represented and their phenologies did not correspond to the vegetation period or to its end in October (Fig. 15).
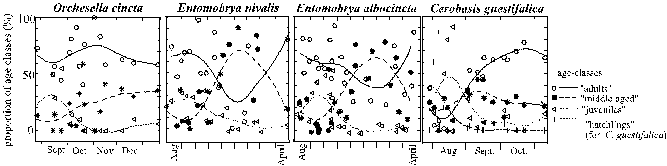
Figure 15: Phenology of the Collembola and of one Psocoptera species plotted as percentages of different age-classes (y axis), during the months when the species was present (x axis). Curves fitted by distance weighted least square smoothing procedure (default settings SYSTAT 3.0). Number of animals per sample (= five subsequently investigated, colonised trees pooled) = For Orchesella cincta: 15, 14, 14, 20, 33, 43, 24, 61, 17, 30, 5, 18, 17, 19, 10, 17, 5; for Entomobrya nivalis: 25, 10, 19, 6, 13, 15, 41, 36, 8, 38, 33, 11, 9, 20, 20, 8, 24, 39; for Entomobrya albocincta: 13, 19, 14, 8, 12, 34, 16, 8, 36, 26, 36, 16, 53, 10, 25, 21, 29, 8, 13, 10, 17, 8, 31, 23, 17; for Cerobasis guestifalica: 15, 20, 22, 50, 25, 11, 95, 29, 30, 27, 14, 16, 17, 16, 10, 9, 15.
3.2.3. Use of moist macroclimates: foraging opportunities for drought- sensitive animals
Expectation: Densities increase during cloudy and rainy weather (rainy = rain + following time of persisting waterfilms on the trunk), during night and during moist, warm seasons. Increases are strongest in the drought-sensitive, juvenile stages.
Observations: D e n s i t i e s : Seasonally, moisture (low saturation deficits) was combined with moderate warm temperature in September and October (Fig. 5, page 26). During these months only Porcellio scaber and Entelecara penicillata increased in abundances (Fig. 12 b, page 37). Moreover, no species was completely restricted to nights or to cloudy/rainy weather (Fig. 12 c) and only Orchesella cincta and Xenillus discrepans significantly increased under both conditions. Reuterella helvimaculata and Porcellio scaber were more frequent nocturnally and during cloudy weather but decreased during rainy weather. Bdella cf semiscuta was probably more frequent during night (Chi² = 3.24, 1 df, considered due to few sampled individuals; the effect of weather conditions could not be tested). Adult Carabodes labyrinthicus were more frequent during moist weather but decreased during night. Entomobrya nivalis, Entomobrya albocincta and Entelecara penicillata did not correspond to the moderate macroclimatic moisture during cloudy as compared to sunny weather. The latter two species as well as juvenile Carabodes even decreased during night. Also Temnostethus gracilis was probably more frequent diurnally (Chi² = 3.66 (1 df) considered due to the few sampled individuals; could not be tested with respect to weather conditions).
A g e c l a s s e s (Fig. 16): Youngest Entomobrya albocincta and Cerobasis guestifalica were more frequent during certain moist macroclimates (cloudy weather and rainy weather, respectively) but decreased during others (night and in Entomobrya albocincta also rainy weather). Orchesella cincta's juveniles were least frequent during moist, rainy weather (Fig. 16). Middle-aged Entomobrya nivalis and Entomobrya albocincta increased significantly during moist, rainy weather. In Orchesella cincta this tendency was present but not significant. Nocturnally, middle-aged animals became more frequent in Entomobrya albocincta and, in tendency, in Entomobrya nivalis and Orchesella cincta. In contrast, middle-aged Cerobasis guestifalica tended to be overabundant during sunny weather (Fig. 16). Proportions of adults did not change diurnally. In Cerobasis guestifalica they were overrepresented during moderate macroclimatic moisture (cloudy weather), whereas in Entomobrya albocincta and Entomobrya nivalis they decreased during cloudy or rainy weather, respectively (Fig. 16).
3.2.4. Use of rainy weather: opportunities to avoid soil soaking
Expectation: Densities should strongly increase during rainy weather (= rain + following time of persisting waterfilms on the trunk). Then, always large parts of the nearby litter layer and also mostly the soil were covered with waterfilms (the ground near solitary trees is not protected from precipitation by a crown canopy layer like in forests). These water films were recognisable by eye or from an immediate wetting of a paper tissue when laid on the ground anywhere near a solitary tree (example from April 25, 1996: the litter, herb layer, bare ground and moss cushions next to all 20 tested solitary trees during a short, slight rain that had not even caused water films on the trunk).
The age class distribution should react in one of the following two ways: (1) either all age-classes are equally effected, since they are equally endangered by lethal adhesion to waterfilms (PRINZING & WIRTZ in press, for adult Entomobrya-Collembola and for Cerobasis guestifalica) and by increased CO2 concentrations.
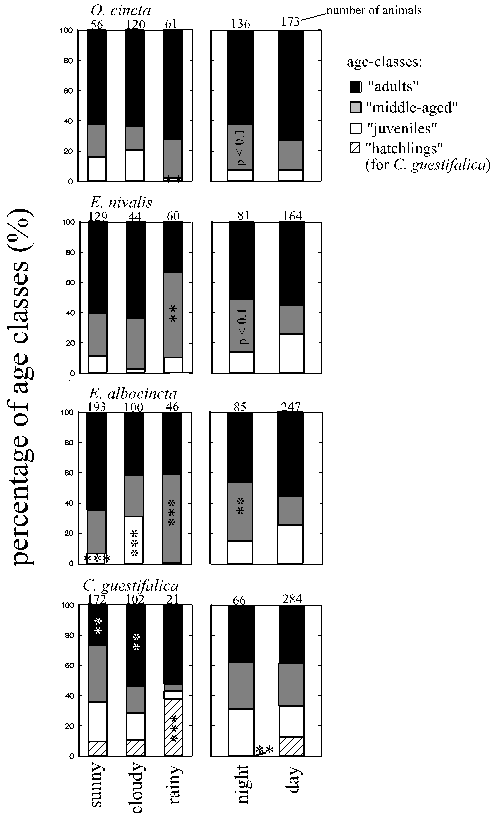
Figure 16: Percentages of age-classes during different weather conditions and daytimes. Age class compositions under each category were compared to the alternative categories: the other daytime, or both other weather conditions pooled; *, **, *** = p < 0.05, 0.01, 0.001 (Chi² test, 2 df in the Collembola, 3 df in Cerobasis guestifalica), marked on the age class with the highest single Chi² value.
(2) Alternatively, either smallest or largest animals might be most effected by soil soaking if mainly small soil pores become inundated (SCHROEDER 1984), or if the inundation does not reach the lower litter layer, where smaller (younger) animals are more frequent due to the smaller cavities (e. g. HAARLOV 1960 for epi- and endogeic microarthropods, STEVENSON & DINDAL 1982 for epigeic spiders).
Observations: D e n s i t i e s : Fig. 12 c (page 38) presents changes of densities during different weather conditions. Obviously, none of the species were restricted or nearly restricted to rainy weather. Juvenile Carabodes, Xenillus discrepans, Cerobasis guestifalica, Reuterella helvimaculata, Porcellio scaber and Entelecara penicillata did not even increase in densities then. The Collembola species Entomobrya nivalis, Entomobrya albocincta and Orchesella cincta and the adult Carabodes labyrinthicus became moderately more frequent during rain. Bdella cf semiscuta and Temnostethus gracilis were not restricted to rainy weather, but statistical testing was prevented by the small number of sampled individuals.
A g e c l a s s e s : Fig. 16 (page 45) compares proportions of age-classes during rain to other weather conditions. Smallest animals were indeed more frequent in Cerobasis guestifalica, whereas in the Collembola the middle-sized animals increased then and in Orchesella cincta the smallest animals became rare.
3.2.5. Use of wind exposure: opportunities to achieve wind dispersal
Expectation: Densities of species that seek for wind dispersal should increase during windy weather and on wind-exposed trunk faces and microrelief zones. On wind-sheltered trunk faces the wind-exposed microrelief zones are used even more (compensation).
Observations (Fig. 12 d, page 38): Only decreases of densities were found in Orchesella cincta, Reuterella helvimaculata (on all scales!), adult Carabodes labyrinthicus, Xenillus discrepans, Temnostethus gracilis, Entelecara penicillata. No significant changes in abundances were found in juvenile Carabodes and Bdella cf semiscuta. Increases but also decreases occurred in Entomobrya albocincta (using windy weather but also wind-sheltered microrelief zones), Cerobasis guestifalica (using wind-exposed microrelief zones but also calm weather) and in Porcellio scaber (using wind-exposed trunk faces but also sheltered microrelief zones and calm weather). Entomobrya nivalis solely increased on wind-exposed microrelief zones, but this did not increase on trunk faces that provided a lower exposure to wind dispersal.
3.3. Use of discrete microhabitats (cryptogam species, crevice types) by corticolous arthropods
According to the previous chapter, the effect of climatic gradients on arthropod distribution is immense, even if the effect of the microhabitat composition is filtered out. These microhabitats will now be looked at more closely. They are discrete entities and thus less regular and less continuously dispersed than climatic gradients, but determine the animals’ food supply and modify their surrounding climate. Microhabitat types can differ in frequency under certain climates. Pseudocorrelations with climate were nevertheless avoided by the applied method of comparison (described in 2.2. and 2.4.3., pages 10 and 17).
3.3.1. Average use of microhabitat types
Average densities of the most frequent arthropod species on different types of microhabitats (Fig. 1, page 9) are presented in Fig. 17. Four patterns of average microhabitat use were especially similar in many species. In Tab. 5 the species are ordered according to their similarity in these four patterns. Obviously, all combinations of patterns were realised. But most species that were more frequent in Evernia prunastri also preferred the other cavity microhabitats (the crevices). Related species (either Collembola or oribatids or Psocoptera or Araneae or Heteroptera) are more similar to each other than to most, but not all other species. In general, the degree of differentiation between microhabitats is very high. Even the various crust-microhabitats were mostly used in significantly different densities, despite the similarity between the crusts of algae and of Lecanora expallens (flat and stiff) and between Lepraria incana and Pertusaria albescens (similar coloration, thickness, consistency and hydrophobic reaction; WIRTH 1980). Lepraria incana ("lep" in Fig. 17) was the most frequently used crust although algae ("al" in Fig. 17) covered much larger areas of the trunks. The increased densities of Reuterella helvimaculata on Lepraria incana were mainly due to male (winged) animals (Chi2 = 4.19, n = 19, p < 0.05, df = 1). The conspicuous red eggs of Bdella cf semiscuta (determined by breeding several in the laboratory) were found mostly on Lepraria incana (175 eggs) and sometimes on Pertusaria albescens (4 eggs). On the crusts of Lecanora expallens 9 out of 13 psychodid flies were found. These nocturnal animals could hardly have discriminated Lecanora expallens optically from other crusts, but probably had used olfactory cues.
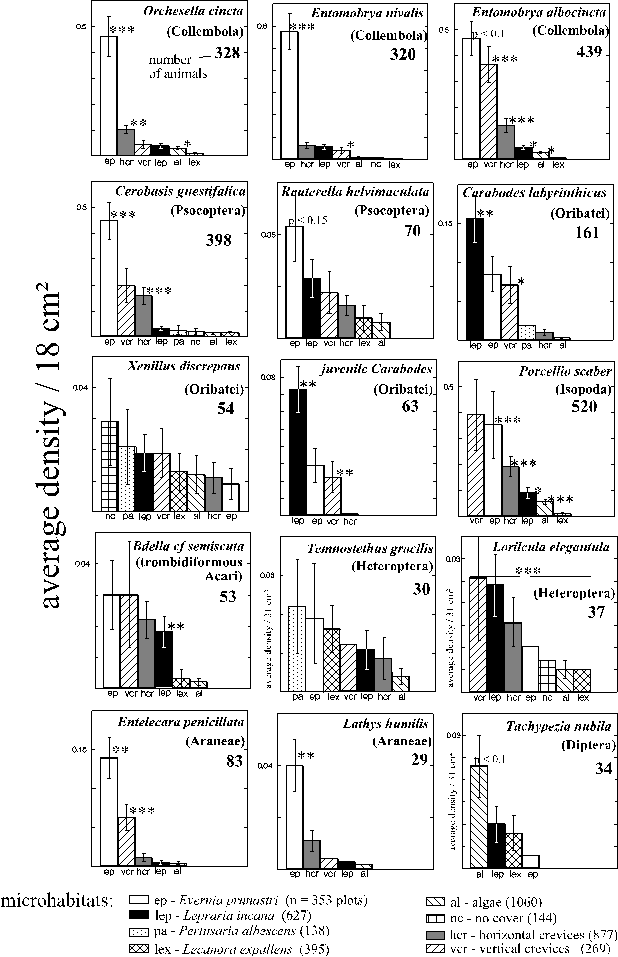
Fig. 17 (legend on next page)
Table 5: Patterns of average densities of the most frequent arthropod species on different microhabitat types (+, - : pattern is, is not realised); species are ordered according to the similarity of the patterns. The complete data are given in Fig. 17. Statements in ( ) are not statistically significant, C. = Collembola, S. = spider, I. = Isopods, T. = trombidiformous mite, O. = oribatid, P. = Psocoptera, A. = anthocorid Heteroptera, L. = loriculid Heteroptera, E. = empidid fly.
|
patterns of microhabitat use: |
||||
|
species in order of similar combina-tions of the microhabitat use patterns |
highest densities on Evernia prunastri (ep in Fig. 17) |
highest densi- ties in all ca- vity microhabi- tats (ep, hcr, vcr in Fig.17) |
densites in horizontal vs vertical crevices (hcr, vcr in Fig. 17) |
most densely colonized crusts (lep, pa, lex, al, nc in Fig.17) |
|
Orchesella cincta L. C. |
+ |
+ |
horizontal > vertical |
L. incana, algae |
|
Lathys humilis Blackwall S. |
+ |
+ |
(horizontal > vertical) |
(L. incana, algae) |
|
Entelecara penicillata Westring S. |
+ |
+ |
vertical > horizontal |
(L. incana, algae) |
|
Entomobrya albocincta Templeton C. |
+ |
+ |
vertical > horizontal |
L. incana |
|
Porcellio scaber Latreille I. |
- |
+ |
vertical > horizontal |
L. incana |
|
Bdella cf semiscuta Thor T. |
- |
+ |
(vertical > horizontal) |
L. incana |
|
Reuterella helvimaculata Enderlein P. |
+ |
- |
vertical @ horizontal |
L. incana |
|
Entomobrya nivalis L. C. |
+ |
almost + |
vertical @ horizontal |
L. incana |
|
Cerobasis guestifalica Kolbe P. |
+ |
+ |
vertical @ horizontal |
(P. albescens, L. incana) |
|
Temnosthetus gracilis Horv. A. |
- |
- |
(vertical @ horizontal) |
(P. albescens) |
|
Xenillus discrepans Grandjean O. |
- |
- |
(vertical > horizontal) |
(no cover, P. albescens) |
|
Carabodes labyrinthicus Michael O. |
- |
- |
vertical > horizontal |
L. incana |
|
juvenile Carabodes O. |
- |
- |
vertical > horizontal |
L. incana |
|
Loricula elegantula Bärensprung L. |
- |
- |
(vertical > horizontal) |
(L. incana) |
|
Tachypezia nubila Meig. E. |
- |
- |
not used |
algae |
Figure 17 (previous page): Average densities of the most frequent arthropod species on the different types of microhabitats (means, + 1 SEM for samples of > 3 animals). Differences were tested between microhabitat types of neighbouring rank (Chi² test of goodness if applicable, otherwise Kolmogorov-Smirnov-test of goodness, *, **, *** = p < 0.05, 0.01, 0.001, respectively). "Juvenile Carabodes" see 3) .
3.3.2. Microhabitat use of different age-classes
The proportions of different age-classes of Collembola and of Cerobasis guestifalica on the five most frequently used microhabitat types are presented in Fig. 18.
Algal crusts were colonised overproportionally by adults in Entomobrya albocincta and probably in Cerobasis guestifalica (not statistically significant, due to the small number of animals that could be sampled on algae). On crusts of Lepraria incana juvenile Entomobrya nivalis were overrepresented. The most even distribution of age-classes was found in the shrub-like thalli of Evernia prunastri. In hori
zontal crevices, the intermediate age class was proportionally most frequent in Cerobasis guestifalica ("juveniles") and in Entomobrya albocincta ("middle-aged" animals), but it was least frequent in Orchesella cincta ("middle-aged" animals). In vertical crevices, proportions of the respective youngest age-classes were increased in Entomobrya nivalis ("juveniles") and in Cerobasis guestifalica ("hatchlings").
Most of the collected spiders had been characterised as either mature (i. e. complete differentiation of the genitalia) or immature. Immature Lathys humilis were overrepresented in horizontal crevices compared to the other colonised cavity microhabitats: Evernia prunastri and vertical crevices (p = 0.014, Fisher test, n = 18). In contrast, immature Entelecara penicillata were slightly overproportional in Evernia prunastri compared to the crevices (Chi2 = 3.5, p < 0.1, df = 1, n = 65).
3.3.3. Effect of climate on microhabitat use
The suitability of microhabitats and the needs of the microarthropods change namely under different climates. The resulting patterns of microhabitat use will be analysed below. The considered climatic variables are differentiated on many scales: from centimetres and a few hours (temperature zonation of the microrelief) to metres (trunk face zonation) and days (weather conditions) or months (temper-ature). The larger scales prevented biases due to climates before the exact moment and next to the exact point at which the individual animal had been sampled. The smaller scales took into account the climatic choices which the respective animal actually had.
3.3.3.1. Univariate analysis:
The three Collembola species, the Psocoptera Cerobasis guestifalica, the adult oribatids of Carabodes labyrinthicus and the Isopod Porcellio scaber were sufficiently frequent for a comparison of microhabitat use during different climates, considering the following microhabitats: Evernia prunastri, Lepraria incana, vertical crevices (except in Entomobrya nivalis), horizontal crevices (except in adult Carabodes labyrinthicus) and algae (except in Entomobrya nivalis and Carabodes labyrinthicus).
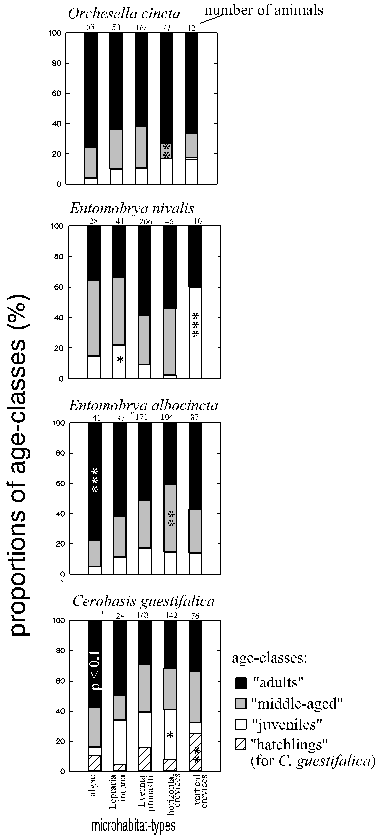
Figure 18: Percentages of age-classes of selected arthropod species on the most frequently colonised microhabitat types. The age class composition on each microhabitat was compared to those on all other microhabitat types pooled. *, **, *** = p < 0.05, 0.01, 0.001 (Chi² test, 2 df in the Collembola, 3 df in Cerobasis guestifalica), marked on the age class with the highest single Chi² value.
Fig. 19 (pages 53-55) presents relative densities during different absolute thermic and hygric conditions (graphs 1-23), within different zonations of temperatures and waterfilms (graphs 24-45) and under different wind impacts (graphs 46-61). The numbers of the graphs (in italics) are referred to in the text. Continuous variables were subdivided into distinct categories that sufficiently represented the range of the variable and also contained enough animals for statistical analysis. A species' microhabitat use was not compared to a climatic category when the calculated expected frequency on at least one of the relevant microhabitat types was less than three.
A real, causal effect of a climate on the microhabitat colonisation was often likely since one or more species interacted similarly with several physiologically equivalent, but mutually independent climates. Examples of such climates are: (i) the temperature of the trunk and the relative upheating of the trunk face and of the microrelief zone compared to the opposite zone, or (ii) radiation due to sunny weather (during the day) and radiation during the daytime as compared to night.
In the text, the results are summarised for each microhabitat type. This is the most comprehensive and the shortest way.
The number of animals under a certain climate is given in the Fig. 19. Numbers of 18 cm² plots searched of the microhabitat types "no cover, algae, Lecanora expallens, Lepraria incana, Evernia prunastri, horizontal crevices, vertical crevices" under =< 0 / 0-7 / 7-14 / > 14 °C are: 7, 61, 16, 39, 22, 62, 17 / 28, 463, 141, 258, 147, 344, 88 / 55, 298, 131, 181, 100, 250, 94 / 51, 234, 107, 148, 82, 217, 69; during = < 66 / > 66 Pascal: 39, 257, 98, 101, 131, 183, 93 / 60, 337, 147, 155, 152, 314, 160; under sunny / cloudy / rainy weather: 64, 411, 159, 224, 153, 377, 138 / 51, 296, 123, 193, 103, 260, 73 / 12, 123, 41, 63, 41, 87, 19; during night / day: 15, 199, 66, 127, 51, 133, 38 / 78, 441, 188, 262, 151, 370, 111; on cooler / warmer trunk faces: 56, 421,139, 257, 133, 368, 117 / 76, 416, 191, 227, 164, 351, 124; on cooler / warmer microrelief zone: 61, 378, 137, 227, 133, 333, 117 / 56, 397, 168, 226, 131, 326, 98; on trunk zones cooler / warmer than the macroclimate: 38, 339, 85, 193, 98, 228, 66 / 65, 420, 191, 226, 156, 377, 130; on trunk face without / with waterfilm: 8, 80, 27, 42, 18, 48, 10 / 6, 101, 21, 56, 34, 60, 13; on wind-sheltered /-exposed microrelief zones on wind-sheltered trunk faces (no vertical crevices considered): 10, 153, 52, 136, 14, 125 / 35, 148, 66, 57, 77, 129; ditto for wind-exposed trunk faces: 15, 155, 42, 133, 17, 125 / 25, 154, 75, 68, 90, 128; durng wind speed of =< 3 / 3 Beaufort: 86, 565, 239, 376, 212, 497, 151 / 49, 384, 137, 195, 120, 316, 96.
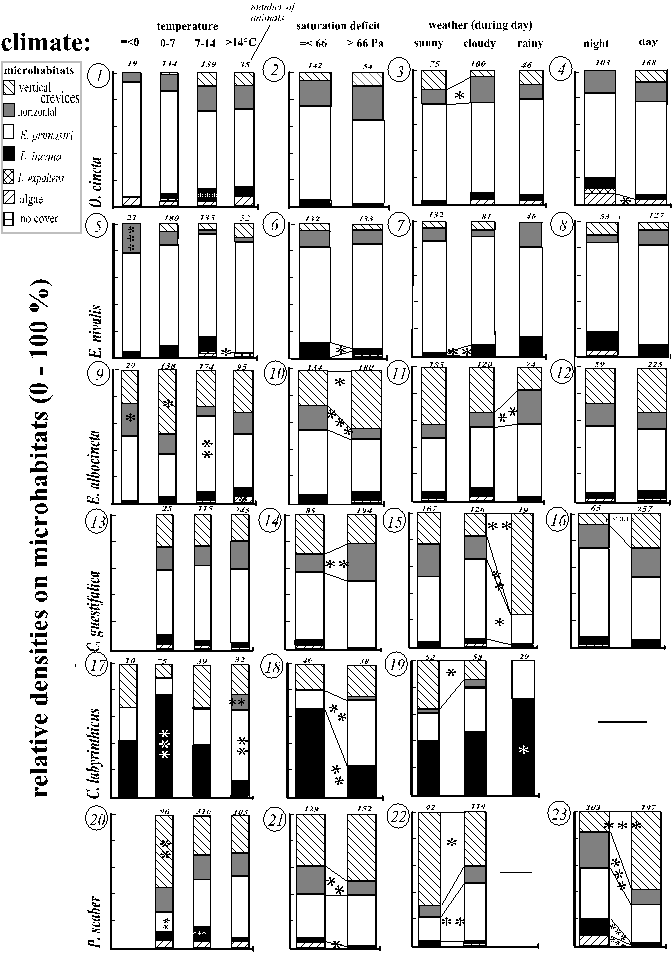
Figure 19 (legend see previous page):
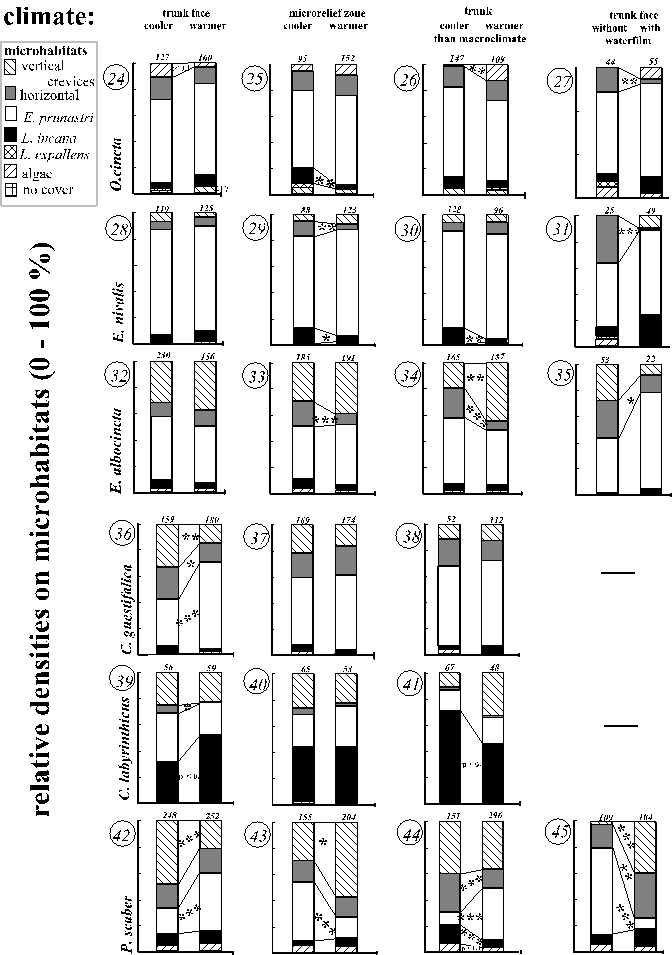
Figure 19 (continued, legend on page 52):
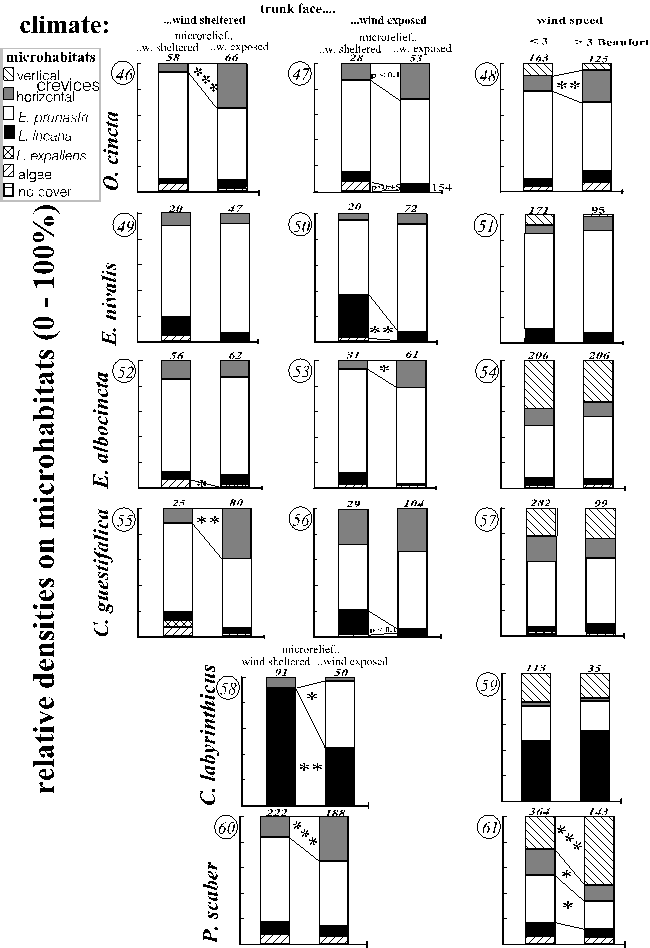
Figure 19 (continued, legend on page 52):
Evernia prunastri was either used constantly (Orchesella cincta, Entomobrya nivalis) or densities increased during xeric climates: (i) moderate or strong heat (Entomobrya albocincta or Carabodes labyrinthicus and Porcellio scaber; Fig. 19: 9, 17, 20), (ii) drought (Carabodes labyrinthicus; Fig 19: 18), (iii) cloudy compared to rainy weather (Cerobasis guestifalica; Fig. 19: 15), (iv) warmer trunk faces (Cerobasis guestifalica, Porcellio scaber; Fig. 19: 36, 42), (v) trunk zones warmer than the macroclimate (Porcellio scaber 19: 44). Rarely, more mesic climates were relevant, too: Porcellio scaber became more frequent during cloudy compared to sunny weather and on cooled microrelief zones (Fig. 19: 22, 43). Further increases corresponded to exposure to wind (Carabodes labyrinthicus; Fig. 19: 58), low wind speed and shelter from waterfilm (Porcellio scaber 19: 61, 45).
On crusts of Lepraria incana many species became more frequent under moderate temperatures (moderately warm: Orchesella cincta, Entomobrya nivalis, Por-cellio scaber, moderately cool: Carabodes labyrinthicus; Fig. 19: 1, 5, 20, 17) and under mesic conditions such as: (i) low saturation deficits and moist weather (Entomobrya nivalis, Carabodes labyrinthicus; Fig. 19: 6, 7, 18, 19), (ii) night (Porcellio scaber ; Fig. 19: 23), (iii) cooler microrelief zones (Orchesella cincta, Entomobrya nivalis; Fig. 19: 25, 29) or (iv) trunk zones cooler than the macroclimate (Entomobrya nivalis, Porcellio scaber, Fig. 19: 30, 44). Carabodes labyrinthicus' distribution corresponded slightly to temperature zonations but with opposite tendencies on different scales (Fig. 19: 39, 41).
Shelter from wind within the microrelief favoured Entomobrya nivalis, probably Cerobasis guestifalica and Carabodes labyrinthicus (Fig. 19: 50, 56, 58). In the former two species this effect was highest where the need for shelter was highest: on the wind-exposed trunk faces. For the latter species this could not be tested. However, the direct significance of the exposure to wind is uncertain, because the macroclimatic wind speed did not have any comparable, not even significant, effect (Fig. 19: 48, 51, 54, 57, 59, 61; see also discriminant function analysis for Evernia prunastri).
Algal crusts were increasingly colonised during mesic climates (not significant patterns are included for species of which only a few animals could be sampled on algae): night (Orchesella cincta, Porcellio scaber and, not significant, Entomobrya nivalis, Fig. 19: 4, 23, 8) and low saturation deficits (Porcellio scaber, Fig. 19: 21). Moreover, algal crusts were rarely used when exposed to wind (Entomobrya albocincta; Fig. 19: 52; not significant: all other species) or to waterfilms (not significant in Orchesella cincta and Entomobrya nivalis; Fig. 19: 27, 31, 45). Hardly any animal of any species had been found during sunny or rainy weather, but the sample size was too small for statistical testing (Fig. 19: 7, 11, 15, 19, 22). Partly opposite pat
terns were only found in Entomobrya albocincta (increase during high temperatures Fig. 19: 9).
Horizontal crevices were also increasingly used during mesic conditions: (i) low saturation deficits (Entomobrya albocincta and Porcellio scaber; Fig. 19: 10, 21, but not Cerobasis guestifalica Fig. 19: 14), (ii) night (Porcellio scaber; Fig. 19: 23), and (iii) cloudy or rainy weather (Orchesella cincta, Entomobrya albocincta, but not Cerobasis guestifalica; Fig. 19: 3, 11, 15), provided that (iv) there was no waterfilm (Collembola: Fig. 19: 27, 31, 35; opposite in Porcellio scaber: Fig. 19: 45; not testable for Cerobasis guestifalica and Carabodes labyrinthicus). Further increases corresponded to coldness: (a) low temperatures (Entomobrya nivalis, E. albocincta but not Carabodes labyrinthicus; Fig. 19: 5, 9, 17); (b) relative cooling on various scales (all species except Orchesella cincta; Fig. 19: 29, 33, 34, 36, 39, 44). Horizontal crevices were increasingly used within wind-exposed microrelief zones by Orchesella cincta, Entomobrya albocincta, Cerobasis guestifalica and Porcellio scaber (Fig. 19: 46, 47, 53, 55, 60). However, exposure of the trunk face to wind intensified this pattern only in Entomobrya albocincta (Fig. 19: 53). During strong wind an equivalent increase was only realised in Orchesella cincta, whereas Porcellio scaber decreased then (Fig. 19: 48, 61).
Vertical crevices were colonised to a greater extent during drought and radiation indicated by: (i) high saturation deficits (Entomobrya albocincta; Fig. 18: 10), (ii) sunny weather (Carabodes labyrinthicus, Porcellio scaber; Fig. 19: 19, 22), (iii) day conditions (Porcellio scaber; and apparently, although not testable due to small samples, Cerobasis guestifalica and Orchesella cincta; Fig. 19: 23, 16, 4), and (iv) upheating on various scales (Orchesella cincta, Entomobrya albocincta, Porcellio scaber; Fig. 19: 26, 34, 43). But an increase during rainy weather was also found in Cerobasis guestifalica (Fig. 19: 15) and an increase under water film cover for Porcellio scaber (Fig. 19: 45). And sometimes relative densities increased under moderately cool temperatures or on cool trunk faces (Entomobrya albocincta, Cerobasis guestifalica, Porcellio scaber Fig. 19: 9, 36, 20, 42).
3.3.3.2. Discriminant function analysis
Despite the diversity of microclimatic interactions in general, the microhabitat use of each species could be largely characterised by a few, simple and well separated climatic complexes: the canonical factors of discriminant function analyses (Tab. 6, page 59). Such canonical factors are calculated in a way that ensures statistical independence. Each canonical factor is dominated by those original variables with the highest 5) canonical loadings (which also reflect the indirect effects of these variables). The absolute value of the loading of dominant variables clearly exceeded each of the other variables for: > .07 to > .56, on average > .22 (in the first canonical factor even > .38). Each factor was dominated by only one, two or rarely three of the 14 variables (except for factor two in Porcellio scaber, Tab. 6). Moreover, the most relevant, first and second factors of most species were well separated from each other due to their different dominant climatic variables (except for Porcellio scaber; Tab. 6). These variables were: (a) wind exposure of the microrelief (first factor in all species); (b) water films (Entomobrya nivalis, Cerobasis guestifalica; moreover moderate loading on the second factor in Entomobrya albocincta, high loadings in the third factors in Porcellio scaber and in Orchesella cincta); (c) heat and drought as reflected by either temperature (Entomobrya nivalis, Carabodes labyrinthicus) or by temperature zonation within the microrelief (Orchesella cincta) or by upheating of the bark compared to the macroclimate (Entomobrya albocincta) or by sunny weather (Entomobrya albocincta, Cerobasis guestifalica, Porcellio scaber, Tab. 6). Interestingly, the well predictable diurnal changes were only relevant in Porcellio scaber. The animals can perceive all mentioned variables either from one point (temperature, weather, waterfilm, daytime) or from gradients of only a few millimetres.
The canonical factors seemed to influence a large part of the species' microhabitat use: (i) all discriminant function analyses were highly significant. (ii) Canonical correlations between the calculated and the real microhabitat use were high, ranging between 0.55 and 0.81 for the different species' first canonical factor and between 0.44 and 0.53 for the second (Tab. 6). (iii) The mathematical assignment of the animals of a species to a microhabitat type was correct in 2.4 to 3.8 as many cases as it would be expected by random (averages of all microhabitat types for the individual arthropod species).
Table 6: Combinations of variables (canonical factors) dominating the microhabitat use according to discriminant function analyses (see Fig. 20). The canonical factors (columns 1 - 4) are mutually independent and highly significant. Numbers indicate correlations of a factor with the original variables. The canonical correlation (last row) indicates the factor's explanatory value. Bold, underlined numbers indicate dominating variables (see text). Variables (units) are: a) = temperature at 2 mm above the trunk (°C), b)/c) = difference in temperature between opposing trunk faces/microrelief zones (°C), d)/e) = strength of temperature gradient (= 0 or 1 when absolute value of b)/c) is < or > 1 °C), f) = warming of trunk boundary layer compared to the macroclimate at 2 m distance (°C), g) = wind speed (Beaufort-scale), h)/i) = wind exposure of the trunk face / microrelief zone (+/- 1; vertical crevices are always situated below the top of wind-exposed microrelief zones and were therefore assigned to 0); j)/k) = sun exposure of trunk face / microrelief zone (+/- 1, 0 for non-sunny weather), l) = trunk face with/without waterfilm during precipitation (+/-1, 0 for non-rainy weather), m) = night/day (0/1), n) = sunny/cloudy/rainy weather (1/2/3). In Carabodes labyrinbthicus sample sizes were only sufficient in four microhabitats and, thus, only three canonical factors were calculated.
|
species:: |
Orchesella cincta |
Entomobrya nivalis |
Entomobrya albocincta |
Cerobasis guestifalica |
Carabodes labyrinthicus |
Porcellio scaber |
|||||||||||||||||||||||
|
canonical factors:: |
1 |
2 |
3 |
4 |
1 |
2 |
3 |
4 |
1 |
2 |
3 |
4 |
1 |
2 |
3 |
4 |
1 |
2 |
3 |
1 |
2 |
3 |
4 |
||||||
|
original variables: |
|||||||||||||||||||||||||||||
|
a) temperature |
.21 |
.29 |
-.34 |
.46 |
-.02 |
.43 |
.17 |
-.21 |
.20 |
-.24 |
-.51 |
-.03 |
-.15 |
.25 |
-.12 |
.19 |
-.26 |
-.73 |
.24 |
-.19 |
.12 |
.04 |
-.63 |
||||||
|
b) D temptf |
.12 |
-.19 |
.02 |
.16 |
-.06 |
-.13 |
.00 |
-.01 |
.08 |
-.07 |
.10 |
.02 |
-.17 |
-.04 |
.05 |
.06 |
-.29 |
.18 |
.12 |
-.16 |
.15 |
.05 |
-.31 |
||||||
|
c) D tempmr |
.02 |
.47 |
.04 |
.04 |
.03 |
.24 |
.36 |
-.21 |
.11 |
-.23 |
-.36 |
-.17 |
.09 |
.24 |
-.04 |
.10 |
.40 |
-.07 |
-.16 |
.18 |
-.08 |
.46 |
.06 |
||||||
|
d) | D temptf | |
-.01 |
.40 |
-.01 |
.27 |
.11 |
.27 |
-.71 |
.18 |
.12 |
-.27 |
.24 |
.05 |
-.06 |
.31 |
.32 |
-.03 |
-.25 |
-.13 |
-.06 |
.13 |
.02 |
-.28 |
.46 |
||||||
|
e) | D tempmr | |
.24 |
.38 |
.17 |
-.53 |
.03 |
.07 |
-.51 |
.12 |
.17 |
-.11 |
.05 |
-.03 |
-.17 |
-.02 |
-.20 |
.12 |
-.43 |
.18 |
.31 |
-.04 |
.00 |
.15 |
-.02 |
||||||
|
f) D tempatm. |
.10 |
.20 |
-.07 |
.32 |
.07 |
.31 |
-.53 |
-.18 |
.13 |
-.60 |
.16 |
.18 |
-.17 |
.17 |
.13 |
.30 |
-.44 |
.13 |
.14 |
.32 |
.15 |
.41 |
-.06 |
||||||
|
g) wind speed |
.35 |
.00 |
-.64 |
.06 |
-.02 |
-.30 |
.26 |
.31 |
.09 |
.05 |
-.20 |
-.27 |
.20 |
.18 |
-.18 |
.07 |
.00 |
.06 |
-.02 |
-.03 |
-.32 |
.15 |
.38 |
||||||
|
h) wind expos.tf |
-.10 |
.04 |
.27 |
.29 |
-.03 |
.10 |
.31 |
.04 |
.11 |
-.04 |
.12 |
-.23 |
.14 |
.35 |
-.33 |
.14 |
-.06 |
.10 |
.00 |
-.14 |
-.34 |
.18 |
.10 |
||||||
|
i) wind expos.mr |
-.70 |
.05 |
-.31 |
.11 |
.80 |
.11 |
.02 |
.15 |
.76 |
-.03 |
.36 |
-.03 |
-.77 |
.16 |
.21 |
-.09 |
-.57 |
-.09 |
-.11 |
-.45 |
.17 |
.23 |
-.10 |
||||||
|
j) sun exposuretf |
.08 |
-.39 |
.00 |
.17 |
.02 |
-.07 |
.12 |
.33 |
.07 |
-.05 |
.26 |
-.22 |
-.16 |
-.28 |
-.17 |
.32 |
-.16 |
.31 |
.32 |
-.24 |
-.14 |
-.19 |
-.19 |
||||||
|
k) sun exposuremr |
-.17 |
.21 |
-.07 |
-.14 |
.06 |
.13 |
.10 |
-.06 |
.16 |
-.12 |
.23 |
-.49 |
-.02 |
-.14 |
.19 |
.63 |
-.10 |
-.17 |
-.06 |
-.34 |
-.34 |
-.06 |
-.11 |
||||||
|
l) waterfilmtf |
-.07 |
.05 |
.53 |
.53 |
-.20 |
.41 |
.07 |
.42 |
.17 |
-.38 |
-.34 |
.30 |
.12 |
.47 |
-.33 |
-.01 |
.06 |
-.02 |
-.11 |
.24 |
-.33 |
.47 |
-.03 |
||||||
|
m) night/day |
-.31 |
.38 |
-.12 |
-.01 |
.25 |
-.10 |
-.12 |
-.12 |
.02 |
-.07 |
.06 |
.36 |
.11 |
.18 |
.44 |
.07 |
-.04 |
.04 |
.23 |
-.49 |
-.39 |
.02 |
-.15 |
||||||
|
n) weather |
.04 |
-.36 |
.14 |
-.14 |
-.24 |
.22 |
.31 |
.12 |
.13 |
.56 |
-.15 |
.01 |
-.05 |
-.42 |
-.20 |
-.06 |
.11 |
.26 |
.31 |
.16 |
.42 |
.07 |
.14 |
||||||
|
canonical correlations: |
.58 |
.48 |
.33 |
.24 |
.80 |
.50 |
.25 |
.23 |
.57 |
.45 |
.26 |
.13 |
.55 |
.45 |
.33 |
.29 |
.81 |
.52 |
.40 |
.60 |
.53 |
.38 |
.25 |
||||||
The use of each microhabitat type is characterised by the first and second canonical factor in Fig. 20 (page 61). The first factor always separated basically different microhabitats such as the shrub-like thalli of Evernia prunastri or the crusts or the crevices or the cavity microhabitats. The crusts of algae and of Lepraria incana were only differentiated from each other along the second canonical factor, just like the vertical and the horizontal crevices.
Evernia prunastri was mainly colonised during climates dominated by wind exposure of the microrelief (most extreme in Carabodes labyrinthicus). This is opposite to most other relevant microhabitat types. Calm, rainy weather and shelters against wind, sun and water film were additionally important for the presence of Porcellio scaber in Evernia prunastri. Heat and waterfilms slightly favoured Entomobrya nivalis (Fig. 20).
The univariate analyses (3.3.3.1.) had only revealed a significant interaction with microrelief wind exposure in Carabodes labyrinthicus. In other species such a univariate effect might have been masked by other variables. Such variables can be recognised in the discriminant function analyses (Tab. 6): they have a strong loading in the same, first canonical factor that is dominated by the microrelief's wind exposure. These are variables which reflect harsh and fluctuating climates (causing strong convective desiccation at the wind-exposed zones): day conditions (Orchesella cincta, Entomobrya nivalis, Porcellio scaber), sunny weather (Entomobrya nivalis), low wind turbulences (Orchesella cincta, Cerobasis guestifalica), heat or upheating (Entomobrya albocincta, Carabodes labyrinthicus, Cerobasis guestifalica), strong microrelief gradients of temperature (Entomobrya albocincta, Cerobasis guestifalica, Carabodes labyrinthicus) and a sun exposed microrelief (Porcellio scaber) (considering the second and third most important variables). In Carabodes laby-rinthicus the secondary effect of heat on the extreme position along the important first factor was probably more relevant than the primary effect of coldness on the only slightly positive position along the less important second factor.
The effect of wind speed of the macroclimate was subordinate (Tab. 6) - just like in univariate analyses (Fig. 19, page 55). Mostly it did not interact with the effect of wind exposure of the microrelief: both variables yielded their highest canonical loadings in different factors. Only in Orchesella cincta and Cerobasis guestifalica did the effects of both wind speed and wind exposure of the microrelief zone interact, namely negatively (Tab. 6, page 59).
The colonisation of Lepraria incana increased during wind shelter by the microrelief and, in Porcellio scaber, also during nocturnal climates. Positions along the canonical factors were always extreme, except in Carabodes labyrinthicus (Fig. 20). Univariate results are confirmed for all species (in Orchesella cincta and
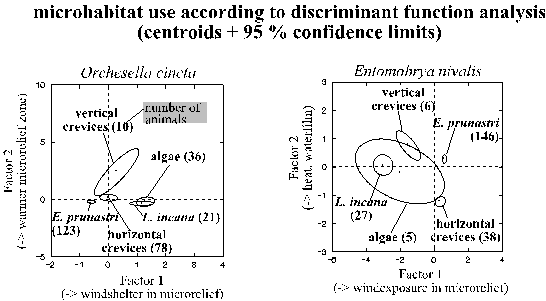
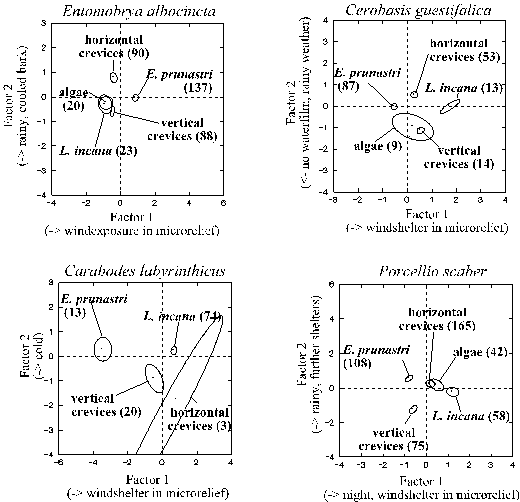
Figure 20: Use of different microhabitat types by the most common arthropod species according to first and second canonical factors of the discriminant function analyses in Table 6. The dominating original variables are noted next to the axes. For animals of each microhabitat type the centroid of distribution, its 95 % confidence limit and the number of sampled individuals are given. The graphs include the complete discriminant spaces.
Entomobrya albocincta the not significant patterns on wind-exposed trunk faces, Fig. 19: 47, 53, page 55).
Algae were also used primarily in wind-sheltered climates. Water films restricted access for Entomobrya nivalis and Cerobasis guestifalica. Further restrictions were due to sunny weather (Cerobasis guestifalica) and day as compared to night (Porcellio scaber). Algae were colonised under extreme conditions in all species, except in Porcellio scaber (Fig. 20). These patterns confirmed the univariate comparisons (Fig. 19) which were sometimes not significant due to the small number of animals sampled on algae.
Horizontal crevices were used under opposite conditions by different species: (a) cold or warm seasons (Entomobrya nivalis or Carabodes labyrinthicus, respectively), (b) sunny weather (Cerobasis guestifalica) or rainy weather on cooled trunk zones (Entomobrya albocincta) and night as compared to day (Porcellio scaber). Entomobrya nivalis did not avoid rainy weather like Cerobasis guestifalica but then required a shelter from water film (Fig. 20). All this confirmed most univariate results (Fig. 19, pages 53-55). The increase of Orchesella cincta under wind exposure according to univariate analysis was more cryptic in the discriminant function analysis, being only reflected by the position relative to algae or Lepraria incana (Fig. 20).
Vertical crevices were mostly used under warm or sunny climates - except in Cerobasis guestifalica which occurred during rainy weather on zones without a waterfilm. Most species used vertical crevices under comparatively extreme conditions, except Carabodes labyrinthicus (Fig. 20). The results basically corresponded to univariate analysis (Fig. 19, pages 53-55). Exceptions: (1) in Orchesella cincta the univariate effect of trunk/macroclimate gradients was replaced multivariately by their physical causes (Tab. 2, page 27), especially by the accumulation of heat in certain microrelief zones; (2) in Carabodes labyrinthicus, the univariate effect of sunny weather appeared multivariately as an effect of heat.
3.3.4. Effect of the patch size on the microhabitat use
The area covered by an epiphyte within a zone of exposure on a trunk hardly influenced the absolute or (more important) the relative densities of arthropods steadily (Fig. 21). Only once in the fourteen cases both relative and absolute densities increased with the dominance of the epiphyte: in Entomobrya albocincta on Evernia prunastri. Additionally, relative densities of Cerobasis guestifalica decreased steadily with increasing dominance of Lepraria incana and of Evernia prunastri. But this was not reflected by the absolute densities (Fig. 21).
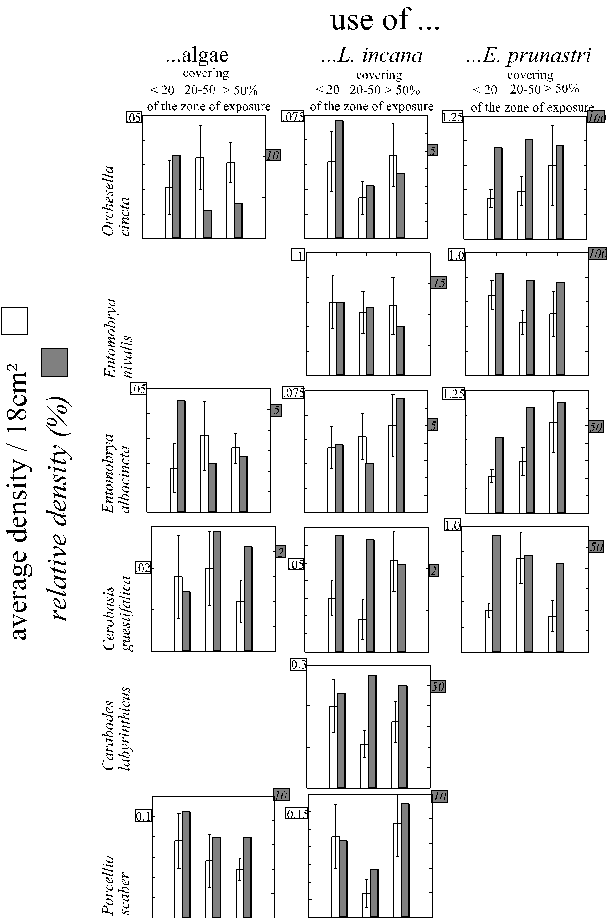
Figure 21: Average densities (+ 1 SEM) and relative densities (definition see Fig 19) of the most frequent arthropod species on each of the frequently used epiphyte species. Microhabitat use almost never changed steadily with the dominance of the epiphyte.
3.4. Morphogenesis of the heavily grazed and wind-exposed
lichen Evernia prunastri
The last chapter revealed that the grazers colonised thalli of Evernia prunastri most densely and also most continuously. The consequences of this distributional pattern for the morphogenesis of the lichens’ peculiar shrub-like thalli shall be investigated in the following. This requires the observation of the lichen’s morphogenesis and its interaction with the environment in general.
3.4.1. Morphogenetic patterns of branches
The tip of a branch developed one ramification per year. Branches mostly grew along their young parts within the last two ramifications, which was observed 155 times compared to only 46 cases of increase in older parts of branches (on photographs from '91/'92, n = 15 sufficiently focused thalli).
Some parts of branches were uniform, flat, even and ramifying isotomous-dichotomously, whereas others showed numerous irregularities in cross-section, growth-layer and branching pattern (Table 7). Asymmetries in cross-sections almost always preceded such variations of growth-layers or branching patterns in space and time and thus probably induced them (Table 8, Fig. 22).
3.4.2. Correlation between arthropod grazing and the growth of branches:
Structure: The structure and regeneration of feeding traces in the cortex and phycobiot layer are documented in Fig. 23. The furrow relief at the ground of feeding traces in Fig. 23 is characteristic for grazing by adult oribatids (PRINZING & WIRTZ in press). Freshly regenerated feeding traces in the cortex and phycobiot-layer were often recognisable as depressions in the branch's surface which were covered by a smooth, intact and clean cortex often showing a furrow-relief. Below this cortex the phycobiot-layer was sometimes not rebuilt (Fig. 23 b).
Regenerated feeding traces along edges of branches developed into corners (Fig. 23 b) with which they were also often found to coincide. The above-mentioned furrows in fresh or regenerated feeding traces of adult oribatids strongly resembled those that were found along many of the corners along edges and also at most of the destroyed tips of branches (Table 7 and Fig. 23). Even the corresponding changes in growth layer (Table 8) could also be observed in the course of regeneration of feeding-traces along edges (Fig. 23 b).
Table 8: Temporal and spatial correlation between the different types of branch cross-sections and the different types of ramifications and changes in growth-layers (presented in Tab. 7). Temporal developments within three months. Numbers of observed branches are given, being recorded on three photographed thalli in 1993 ("(1)") and on 18 thalli in 1991/'92 ("(2)"). All results are highly significant (p < 0.01; Fisher-test for ramification patterns, Chi2-test for growth-layers).
...occured in front of a younger
branch with ...developed ... developed part of the branch with
cross-section ramification-pattern growth-layer growth-layer
like like like like
A C A B A B A B
A... 7 0 48 2 47 2 30 0
B... 5 34 6 40 16 9
C... 0 9 (1) (2) (1)
(see Tab. 7) (1)
Figure 22: Distribution and development of different types of branch-morphology: Anisotomous ramifications (small triangles) and corners along edges of branches (arrow-heads) were proportionally more common on the right face (wind-sheltered), whereas isotomous-dichotomous ramifications (white spots) and rounded edges of branches were proportionally more common on the left face (wind-exposed). Changes in inclination and orientation within three months (January to April 1993) are demonstrated exemplary in the lower right corner (indicated by thick arrows). They only occurred along corners of branch-edges. Small outgrowths on top of large, anisotomous branches are lobuli.
Distribution within a thallus: Along the wind-exposed and the upper faces of the photographed thalli the branches were almost ungrazed. Here, the proportion of branches with an irregular growth pattern (Table 7) was significantly smaller than along the more sheltered thallus faces (Chi2 = 4.03, p < 0.05, n = 91 branches in 5 thalli photographed in 1993, Fig. 22).
Artificial injury, "simulated grazing", induced changes in growth layer in 13 out of 14 cases (Fig. 24), whereas this was only observed in two out of the remaining 20 non-manipulated parts of photographed branches with rounded edges (Chi2 = 19.7, p < 0.01).
3.4.3. Correlations between climatic exposure, the growth of branches and the growth form of whole Evernia prunastri thalli
Increase and shape of differently exposed thalli: An increase in size at the tips of branches that were not sheltered from wind by the surrounding bark relief or by thalli was observed only 49 times in comparison with 106 times under wind-sheltered conditions (photographs from '91/'92, 15 thalli, Fig. 25). More increase in sheltered parts of branches was found in all five sufficiently focused photographed trunk-areas (p < 0.05, sign-test, one-tailed). Moreover, branches were more densely
Figure 23: Branch with feeding traces (a, January 1993) and the subsequent regeneration and changes in growth layer (b, until April 1993). Right end of branch in cross-section indicating C = cortex, P = phycobiot layer, M = medulla layer. a: In feeding traces (indicated 1 - 5) cortex and phycobiot layer were eroded and the white medulla was exposed. The same was found at a rupture-zone (6). The ground of many feeding traces was covered with furrows (4 and left part of 2). Outline and cross-section (right) appeared symmetrical, the growth layer was even and constant. b: Many feeding traces were covered by a cortex already (2, 3, 4), but partially no phycobiot layer was regenerated (3 and 4, at the latter the light underside of the branch now reached far up on the edge). Even after regeneration shallow hollows and furrows persisted in the branch's relief; in the cross-section a corner along the edge did persist (S). The growth layer corresponded to the new asymmetry of the cross-section and inclined (large, grey arrows).
Figure 24: Four examples of development of branches with natural ( ) or artificially induced ( ) corners along edges or with intact, rounded edges ( ) between January 1993 (left branch) and April '93 (right branch). The orientation and inclination of branches is indicated by their darkness (frontal positions are lighter). Branches almost always inclined in growth layer along the natural or induced corners. Otherwise growth layers were almost always unchanged.
packed at wind-exposed thallus faces (Wilcoxon-Test, two-tailed, p < 0.02, photos of n = 7 sufficiently focused trunk-areas containing 434 branches). As mentioned above, also the proportion of branches with irregular growth pattern was significantly smaller on the wind-exposed than on the more sheltered thallus faces.
The growth form of complete thalli corresponded to differences in each of the following gradients of wind exposure (evidence for exposure is given in 2.3., page 14) (a) within the microrelief, (b) between different faces of a trunk and (c) at different trunk heights: More exposed thalli appeared to be more dense and more aerodynamic (recognised on each of the 56 trees at 24 sites on > 70 % of the thalli that did not provide the animals with a strong algal epibiosis as alternative food, Fig. 25).
Figure 25: Growth and rupture of branches at differently exposed microsites, August '91 (A) until January '92 (B). On a small scale there was a high diversity of different thallus growth forms. Among them mainly those in January (B) matched the wind exposure in the microrelief very well: at the left and front faces branches were directed lee-, down- or trunkwardly (demonstrated by arrows), resulting in a windproof shape of the thallus. Increase at tips of branches from (A) to (B), was smaller in wind-exposed zones (signature "+1") than in wind-sheltered zones ("-1"). Detachment (from A to B; signature "2"), only came upon such branches that grew on wind-exposed faces in a very loose or wind-opposed manner (in A).
Sun-light is distributed completely differently on the trunk surface than wind (see 2.3., page 14), thus sun-light exposure did not match any of the above-mentioned patterns of thallus growth.
Neither were any of the above-mentioned changes in growth forms found to be correlated with crown-drainage zones on the trunk where precipitation is most intense. Although, sometimes a luxuriant, hanging growth of thalli was striking here.
Mechanical effects of wind: Falling off the bark was the most common cause of death to be observed for Evernia prunastri in the area of investigation (Fig. 25). This happened mainly during strong wind and simultaneous precipitation, thus by windfall. And in fact, detached parts of thalli were demonstrated to have had a more wind-susceptible loose, unaerodynamic growth form compared to undetached neighbouring parts of thalli (confirmed in 47 out of 52 observations in '91/'92 in 11 trunk-areas, Fig. 25). Moreover, growth patterns of branches that became a victim of windfall were more regular (see Table 7) than in neighbouring, intact ones (65 out of 91 observations). Also all of the 88 thalli collected additionally on the ground near trunks were rather regular in growth pattern.